
Registration No. 333-
As filed with the Securities and Exchange Commission on October 14, 2014.
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
Under
The Securities Act of 1933
INOGEN, INC.
(Exact name of registrant as specified in its charter)
|
Delaware |
5960 |
33-0989359 |
|
(State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
326 Bollay Drive
Goleta, California 93117
(805) 562-0500
(Address, including ZIP code, and telephone number, including area code, of registrant’s principal executive offices)
Raymond Huggenberger
326 Bollay Drive
Goleta, California 93117
(805) 562-0500
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
|
Martin J. Waters Daniel R. Koeppen Wilson Sonsini Goodrich & Rosati, Professional Corporation 633 West Fifth Street, 15th Floor Los Angeles, CA 90071 Telephone: (323) 210-2900 Facsimile: (866) 974-7329 |
|
Charles K. Ruck B. Shayne Kennedy Latham & Watkins LLP 650 Town Center Drive, 20th Floor Costa Mesa, CA 92626-1925 Telephone: (714) 540-1235 Facsimile: (714) 755-8290 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act, as amended, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer |
|
¨ |
|
Accelerated filer |
|
¨ |
|
|
|
|
|
|||
|
Non-accelerated filer |
|
x (Do not check if a smaller reporting company) |
|
Smaller reporting company |
|
¨ |
CALCULATION OF REGISTRATION FEE
|
Title of each class of securities to be registered |
|
Proposed offering price |
|
Amount of registration fee |
|
Common Stock, $0.001 par value per share |
|
$50,000,000 |
|
$5,810 |
|
(1) |
Estimated solely for the purpose of computing the amount of the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended. |
|
(2) |
Includes the aggregate offering price of additional shares that the underwriters have the option to purchase pursuant to an option to purchase additional shares. |
The registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment that specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to such Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. The selling stockholders may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and is it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
Subject to completion, dated October 14, 2014
Preliminary Prospectus
shares

Common Stock
The selling stockholders identified in this prospectus are offering shares of common stock of Inogen, Inc. We will not receive any proceeds from the sale of shares of our common stock by the selling stockholders. Inogen, Inc. is not offering any of the shares to be sold in the offering contemplated by this prospectus.
Our common stock is listed on the NASDAQ Global Select Market under the symbol “INGN.” On October 10, 2014, the last reported sale price of our common stock on the NASDAQ Global Select Market was $20.45 per share.
We are an “emerging growth company,” as that term is defined in the Jumpstart Our Business Startups Act of 2012 and, as such, have elected to comply with certain reduced public company reporting requirements for this prospectus and future filings.
Investing in our common stock involves a high degree of risk. See “Risk factors” beginning on page 10.
|
|
|
Per |
|
|
Total |
|
||
|
Public offering price |
|
$ |
|
|
|
$ |
|
|
|
Underwriting discounts and commissions(1) |
|
$ |
|
|
|
$ |
|
|
|
Proceeds to selling stockholders |
|
$ |
|
|
|
$ |
|
|
(1) See “Underwriting” for additional disclosure regarding underwriting discounts, commissions and estimated offering expenses.
The selling stockholders have granted the underwriters an option to purchase up to an additional shares of common stock at the offering price less the underwriting discount. We will not receive any of the proceeds from the shares of common stock sold by the selling stockholders pursuant to any exercise of the underwriters' option to purchase additional shares.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the shares to purchasers on or about , 2014.
J.P. Morgan
William Blair
|
Leerink Partners |
|
Needham & Company |
Prospectus dated , 2014

Table of contents
|
|
|
Page |
|
|
|
1 |
|
|
|
10 |
|
|
|
32 |
|
|
|
33 |
|
|
|
33 |
|
|
|
34 |
|
|
|
35 |
|
|
|
36 |
|
|
|
40 |
|
|
|
57 |
|
|
|
69 |
|
|
|
71 |
|
|
|
73 |
|
|
|
77 |
|
Material U.S. federal income tax consequences to non-U.S. holders of common stock |
|
79 |
|
|
|
82 |
|
|
|
86 |
|
|
|
86 |
|
|
|
86 |
|
|
|
87 |
Neither we, the selling stockholders nor the underwriters have authorized anyone to provide any information other than that contained in this prospectus or in any free writing prospectus prepared by or on behalf of us or to which we have referred you. Neither we nor the selling stockholders take responsibility for, or can provide any assurance as to the reliability of, any other information that others may give you. This prospectus is an offer to sell only the shares offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. You should assume that the information appearing in this prospectus is accurate only as of the date on the front cover of this prospectus, regardless of the time of delivery of this prospectus or any sale of our common stock. Our business, financial condition, results of operations and prospects may have changed since that date.
For investors outside of the United States: Neither we, the selling stockholders nor the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than the United States. Persons outside of the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of common stock and the distribution of this prospectus outside of the United States.
-i-
The items in the following summary are described in more detail later in this prospectus or incorporated by reference into this prospectus from our Annual Report on Form 10-K for the year ended December 31, 2013, our Quarterly Report on Form 10-Q for the quarter period ended June 30, 2014 and our other filings with the Securities and Exchange Commission listed in the section of the prospectus entitled “Incorporation by reference.” This summary provides an overview of selected information and does not contain all of the information you should consider before buying our common stock. Therefore, you should read the entire prospectus carefully, especially the “Risk factors” section beginning on page 10 and our financial statements and the related notes incorporated by reference into this prospectus from our Annual Report on Form 10-K for the year ended December 31, 2013 and our Quarterly Report on Form 10-Q for the quarter ended June 30, 2014, before deciding to invest in our common stock. In this prospectus, unless the context otherwise requires, references to “we,” “us,” “our” or “Inogen” refer to Inogen, Inc.
Overview
We are a medical technology company that develops, manufactures and markets innovative portable oxygen concentrators used to deliver supplemental long-term oxygen therapy to patients suffering from chronic respiratory conditions. Traditionally, these patients have relied on stationary oxygen concentrator systems for use in the home and oxygen tanks or cylinders for mobile use, which we call the delivery model. The tanks and cylinders must be delivered regularly and have a finite amount of oxygen, which requires patients to plan activities outside of their homes around delivery schedules and a finite oxygen supply. Additionally, patients must attach long, cumbersome tubing to their stationary concentrators simply to enable mobility within their homes. Our proprietary Inogen One systems concentrate the air around the patient to offer a single source of supplemental oxygen anytime, anywhere with a portable device weighing approximately 4.8 or 7.0 pounds. Our Inogen One G3 and G2 have up to 4.5 and 5 hours of battery life, respectively, with a single battery and can be plugged into an outlet when at home, in a car, or in a public place with outlets available. Our systems reduce the patient’s reliance on stationary concentrators and scheduled deliveries of tanks with a finite supply of oxygen, thereby improving patient quality of life and fostering mobility.
Although portable oxygen concentrators represent the fastest-growing segment of the Medicare oxygen therapy market, we estimate based on 2012 Medicare data that patients using portable oxygen concentrators represent approximately 4% to 5% of the total addressable oxygen market in the United States. Based on 2012 industry data, we were the leading worldwide manufacturer of portable oxygen concentrators, as well as the largest provider of portable oxygen concentrators to Medicare patients, as measured by dollar volume. We believe we are the only manufacturer of portable oxygen concentrators that employs a direct-to-consumer strategy in the United States, meaning we market our products to patients, process their physician paperwork, provide clinical support as needed and bill Medicare or insurance on their behalf. To pursue a direct-to-consumer strategy, our manufacturing competitors would need to meet national accreditation and state-by-state licensing requirements and secure Medicare billing privileges, as well as compete with the home medical equipment providers that many rely on across their entire homecare business.
We believe our direct-to-consumer strategy has been critical to driving patient adoption of our technology. Other portable oxygen concentrator manufacturers access patients by selling through home medical equipment providers, which we believe are disincentivized to encourage adoption of portable oxygen concentrators. In order to facilitate the regular delivery and pickup of oxygen tanks, home medical equipment providers have invested in geographically dispersed distribution infrastructure consisting of delivery vehicles, physical locations and delivery personnel within each area. Because portable oxygen concentrators eliminate the need for a physical distribution infrastructure, but have higher initial equipment costs than the delivery model, we believe converting to a portable oxygen concentrator model would require significant restructuring and capital investment for home medical equipment providers. Our direct-to-consumer marketing strategy allows us to sidestep the home medical equipment channel, appeal to patients directly and capture both the manufacturing and provider margin associated with long-term oxygen therapy. We believe our ability to capture this top-to-bottom margin, combined with our portable oxygen concentrator technology that eliminates the need for the service and infrastructure costs associated with the delivery model, gives us a cost structure advantage over our competitors.
Since adopting our direct-to-consumer strategy in 2009 following our acquisition of Comfort Life Medical Supply, LLC, which had an active Medicare billing number but few other assets and limited business activities, we have directly sold or rented our Inogen One systems to more than 40,000 patients, growing our revenue from $10.7 million in 2009 to $75.4 million in 2013. In 2013, 22.2% of our revenue came from our international markets and 40.5% of our revenue came from oxygen rentals. Our percentage of rental revenue increased from 35.8% in 2011, increasing our proportion of recurring revenue. Additionally, we have increased our gross margin from 48.0% in 2011, to 49.3% in 2012, and to 51.7% in 2013, primarily due to the change in sales mix toward direct-to-consumer from provider sales, improving system reliability, reducing material cost per system and lowering overhead cost per system. Our net loss was $2.6 million in 2009 transitioning to net income of $25.4 million in 2013. Adjusted net income excluding a one-time tax benefit was $3.6 million in 2013.
-1-
Our market
Overview of oxygen therapy market
We believe the current total addressable oxygen therapy market in the United States is approximately $3 billion to $4 billion, based on 2012 Medicare data and our estimate of the ratio of the Medicare market to the total market. We estimate that more than 2.5 million patients in the United States and more than 4.5 million patients worldwide use oxygen therapy, and more than 60% of oxygen therapy patients in the United States are covered by Medicare. The number of oxygen therapy patients in the United States is projected to grow by approximately 7% to 10% per year between 2013 and 2019, which we believe is the result of earlier diagnosis of chronic respiratory conditions, demographic trends and longer durations of long-term oxygen therapy.
Long-term oxygen therapy has been shown to be a cost-efficient and clinically effective means to treat hypoxemia, a condition in which patients have insufficient oxygen in the blood. Hypoxemic patients are unable to convert oxygen found in the air into the bloodstream in an efficient manner, causing organ damage and poor health. Chronic obstructive pulmonary disease, or COPD, is a leading cause of hypoxemia. Approximately 70% of our patient population has been diagnosed with COPD, which we believe is reflective of the long-term oxygen therapy market in general. Industry sources estimate that 24 million people in the United States suffer from COPD, of which one-half are undiagnosed.
According to our analysis of 2011 and 2012 Medicare data, approximately two-thirds of U.S. oxygen users require ambulatory oxygen and the remaining one-third require only stationary or nocturnal oxygen. Clinical data has shown that ambulatory patients that use oxygen twenty-four hours a day, seven days a week, or 24/7, regardless of whether such patients rely on portable oxygen concentrators or the delivery model, have approximately two times the survival rate and spend at least 60% fewer days annually in the hospital than non-ambulatory 24/7 patients. Of the ambulatory patients, we estimate that approximately 85% rely upon the delivery model, which has the following disadvantages:
|
• |
limited flexibility outside the home, dictated by the finite oxygen supply provided by tanks and cylinders and dependence on delivery schedules; |
|
• |
restricted mobility and inconvenience within the home, as patients must attach long, cumbersome tubing to a noisy stationary concentrator to move within their homes; |
|
• |
products are not cleared for use on commercial aircraft and cannot plug into a vehicle outlet for extended use; and |
|
• |
high costs driven by the infrastructure necessary to establish a geographically diverse distribution network to serve patients locally, as well as personnel, fuel and other costs, which have limited economies of scale and generally increase over time. |
Portable oxygen concentrators were developed in response to many of the limitations associated with traditional oxygen therapy. Portable oxygen concentrators are designed to offer a self-replenishing, unlimited supply of oxygen that is concentrated from the surrounding air and to operate without the need for oxygen tanks or regular oxygen deliveries, enhancing patients independence and mobility. Additionally, because portable oxygen concentrators do not require the physical infrastructure and service intensity of the delivery model, we believe portable oxygen concentrators can provide long-term oxygen therapy with a lower cost structure. Despite the ability of portable oxygen concentrators to address many of the shortcomings of traditional oxygen therapy, we estimate based on 2012 Medicare data that the amount spent by patients with portable oxygen concentrators represents approximately 5% to 6% of total oxygen therapy spend. We believe the following has hindered the market acceptance of portable oxygen concentrators:
|
• |
to obtain portable oxygen concentrators, patients are dependent on home medical equipment providers, which have made significant investments in the physical distribution infrastructure to support the delivery model and which we believe are therefore disincentivized to encourage adoption of portable oxygen concentrators; |
|
• |
constrained manufacturing costs of conventional portable oxygen concentrators, driven by home medical equipment provider preference for products that have lower upfront equipment cost; and |
|
• |
limitations of conventional portable oxygen concentrators, including bulkiness, poor reliability and lack of suitability beyond intermittent or travel use. |
Our solution
Our Inogen One systems provide patients who require long-term oxygen therapy with a reliable, lightweight single solution product that improves quality of life, fosters mobility, and eliminates dependence on both oxygen tanks and oxygen cylinders as well as stationary concentrators. We believe our direct-to-consumer strategy increases our ability to effectively develop, design and market our Inogen One solutions, as it allows us to:
|
• |
drive patient awareness of our portable oxygen concentrators through direct marketing, sidestepping the home medical equipment channel that other manufacturers rely upon across their homecare businesses, and that is incentivized to continue to service oxygen patients through the delivery model; |
-2-
|
• |
capture the manufacturer and home medical equipment provider margins, allowing us to focus on the total cost of the solution and to invest in the development of product features instead of being constrained by the price required to attract representation from a distribution channel. For example, we have invested in features that improve patient satisfaction, product durability, reliability and longevity, which increase the cost of our hardware, but reduce the total cost of our solution by reducing our maintenance and repair cost; and |
|
• |
access and utilize direct patient feedback in our research and development efforts, allowing us to innovate based on this feedback and stay at the forefront of patient preference. For example, we have integrated a double battery into our product offering based on direct patient feedback. |
We believe the combination of our direct-to-consumer strategy with our singular focus on designing and developing oxygen concentrator technology has created the best-in-class portfolio of portable oxygen concentrators. Our two current product offerings, the Inogen One G3 and Inogen One G2, at approximately 4.8 and 7.0 pounds, respectively, are amongst the most lightweight portable oxygen concentrators on the market. We believe our Inogen One solutions offer the following benefits:
|
• |
Single solution for home, ambulatory, travel and nocturnal treatment. We believe our Inogen One solutions are the only portable oxygen concentrators marketed as a single solution, by which we mean a patient can use our Inogen One systems as their only supplemental oxygen source with no need to also use a stationary concentrator regularly. Our compressors are specifically designed to enable our patients to run our portable oxygen concentrators 24/7, whether powered by battery or plugged into an outlet at home or in a car while the battery is recharging. |
|
• |
Reliability. We have made product performance a priority and have improved reliability with each generation. For example, we have introduced patented air-dryer and patent-pending user-replaceable sieve beds to our products, which have improved product performance and, as a result, patient satisfaction. Reliability is not only critical to patient satisfaction, but also cost management, as our minimal physical infrastructure makes product exchanges more costly to us than providers with greater local physical infrastructure. |
|
• |
Effective for nocturnal use. Our Intelligent Delivery Technology enables our portable oxygen concentrators to provide consistent levels of oxygen during sleep despite decreased respiratory rates. As a result, patients can rely on the Inogen One G3 and Inogen One G2 portable oxygen concentrators overnight while sleeping. |
|
• |
Unparalleled flow capacity. Our 4.8 pound Inogen One G3 has at least 50% more flow capacity than other sub-5 pound portable oxygen concentrators, and our 7.0 pound Inogen One G2 has at least 15% more flow capacity than other sub-10 pound portable oxygen concentrators. |
|
• |
User friendly features. Our systems are designed with multiple user friendly features, including long battery life and low noise-levels in their respective weight categories. |
Our strengths
We believe our products and business model position us well to compete not only against other oxygen device manufacturers, but also to increase our share of the overall oxygen therapy market. We believe we have the following advantages relative to both traditional oxygen therapy providers and other oxygen device manufacturers:
|
• |
Attractive economic model. Our non-delivery model allows us to receive a premium monthly Medicare reimbursement for deployment of our devices to oxygen patients versus the delivery model. Standard Medicare reimbursement for ambulatory patients using the delivery model is $208.21 per month versus $229.87 per month for our portable oxygen concentrator model, representing a premium of $21.66 per month. A similar premium was maintained in the round one recompete ($19.09 per month) and in the round two ($23.30 per month) competitive bidding areas. In addition, we believe our portable oxygen concentrator technology and direct-to-consumer strategy allow us to provide our solutions through a more efficient cost structure. The delivery model requires ongoing gaseous or liquid oxygen container refills and regular home deliveries with accompanying costs, while our portable oxygen concentrator non-delivery model eliminates oxygen container refills and regular deliveries of oxygen containers and their associated costs. Following the first two rounds of competitive bidding and the round one recompete, we retained access to approximately 90% of the U.S. long-term oxygen therapy market, with the majority of contracts through mid-2016, while many providers were priced out of this market. |
|
• |
Direct-to-consumer capabilities. We believe our direct-to-consumer strategy enables patient access and retention as well as innovation and investment in our product portfolio. Pursuing a direct-to-consumer strategy requires national accreditation, state-by-state licensing and Medicare billing privileges. We are unaware of any manufacturing competitor that currently markets on a direct-to-consumer basis, and so we do not believe any of these manufacturers possesses the necessary qualification to do so. If any of our manufacturing competitors were to pursue a direct-to-consumer strategy, they would risk negative reaction from the home medical equipment providers that sell their other homecare products, which generally represent significantly larger portions of their businesses than oxygen therapy products. |
-3-
|
• |
Commitment to customer service. We are focused on providing our patients with the highest quality of customer service. We guide them through the reimbursement and physician paperwork process, perform clinical titration and offer 24/7 telephone support, which includes clinical support as required. We have a sustained patient satisfaction rating of approximately 95%, as measured by our customer satisfaction surveys. |
|
• |
Patient-friendly, single-solution, sub-5 and sub-10 pound portable oxygen concentrators. Our Inogen One G3 and Inogen One G2 portable oxygen concentrators are sub-5 and sub-10 pound portable oxygen concentrators that can operate reliably and cost-effectively to service long-term oxygen therapy patients on a 24/7 basis, similar to a stationary oxygen concentrator or replacement portable oxygen concentrators. We believe the technology in our Inogen One portable oxygen concentrators is effective for nocturnal use, allowing patients to receive oxygen therapy around the clock from a single device. |
|
• |
Commitment to research and development and developing intellectual property portfolio. We have a broad patent portfolio relating to the design and construction of our oxygen concentrators and system optimization. Additionally, we have made significant investments in research and development and have a robust product pipeline of next-generation oxygen concentrators. |
|
• |
Management team with proven track record and cost focus. Our management team has built our direct-to-consumer capabilities and launched our two current primary product offerings, Inogen One G2 and Inogen One G3. We continue to realize meaningful product manufacturing cost savings of approximately 40% from 2009 to 2013 as a result of management’s improvements in design, sourcing and reliability, as well as higher production volumes. |
|
• |
Revenue growth, profitability and recurring revenue. We have grown our revenue from $10.7 million in 2009 to $75.4 million in 2013, representing a continuous annual growth rate of 47.8%. In 2013, our recurring rental revenue represented 40.5% of revenues. Our net income was $25.4 million after a one-time tax benefit of $21.8 million, or $3.6 million before the $21.8 million benefit compared to a net loss of $2.6 million in 2009. |
Our strategy
Our goal is to design, build and market oxygen solutions that redefine how oxygen therapy is delivered. To accomplish this goal, we will continue to invest in our product offerings and our commercial infrastructure to:
|
• |
expand our sales and marketing channels, including more internal and physician-based salespeople, increased direct-to-consumer advertising and greater international distribution; |
|
• |
develop innovative products, including next-generation oxygen concentrators and other innovations that improve quality of life; |
|
• |
secure contracts with private payors and Medicaid in order to become in-network with non-Medicare payors, which represent at least 30% of our home oxygen therapy patients, and we believe represent a younger and more active patient population; and |
|
• |
continue to focus on cost reduction through scalable manufacturing, reliability improvements, asset utilization and service cost reduction. |
Risks associated with our business
Our ability to implement our business strategy is subject to numerous risks that you should be aware of before making an investment decision. These risks are described more fully in the section entitled “Risk factors” immediately following this prospectus summary. These risks include, among others:
|
• |
A significant majority of our customers have health coverage under the Medicare program, and recently enacted and future changes in the reimbursement rates or payment methodologies under Medicare and other government programs have and could continue to materially and adversely affect our business and operating results; |
|
• |
The implementation of the competitive bidding process under Medicare could negatively affect our business and financial condition; |
|
• |
We face intense national, regional and local competition and if we are unable to compete successfully, it could have an adverse effect on our revenue, revenue growth rate, if any, and market share; |
|
• |
If we are unable to continue to enhance our existing products, develop and market new products that respond to customer needs and preferences and achieve market acceptance, we may experience a decrease in demand for our products and our business could suffer; |
|
• |
If we fail to expand and maintain an effective sales force or successfully develop our international distribution network, our business, financial condition and operating results may be adversely affected; and |
|
• |
If we are unable to secure and maintain patent or other intellectual property protection for the intellectual property used in our products, we will lose a significant competitive advantage. |
-4-
Corporate history and information
We were incorporated in Delaware in November 2001. Our principal executive offices are located at 326 Bollay Drive, Goleta, California 93117. Our telephone number is (805) 562-0500. Our website address is www.inogen.com. Information contained on the website is not incorporated by reference into this prospectus, and should not be considered to be part of this prospectus.
We use “Inogen,” “Inogen One,” “Inogen One G2,” “Inogen One G3,” “oxygen.anytime.anywhere” and other marks as trademarks in the United States and other countries. This prospectus contains references to our trademarks and service marks and to those belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos, artwork and other visual displays, may appear without the ® or ™ symbols, but such references are not intended to indicate in any way that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other entities’ trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, any other entity.
Emerging growth company status
We are an “emerging growth company,” as that term is defined in Section 2(a) of the Securities Act of 1933, as amended, or the Securities Act, as modified by the Jumpstart Our Business Startups (JOBS) Act of 2012. For as long as we qualify as an emerging growth company, we have taken, and may continue to take, advantage of certain exemptions from various reporting requirements that are applicable to other public companies that do not qualify as emerging growth companies, including, without limitation, not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002, reduced disclosure obligations relating to executive compensation and exemptions from the requirements of holding advisory “say-on-pay,” “say-when-on-pay,” and “golden parachute” executive compensation votes.
Under the JOBS Act, we will remain an emerging growth company until the earliest of:
|
• |
the last day of the fiscal year during which we have total annual gross revenues of $1 billion or more; |
|
• |
the last day of the fiscal year following the fifth anniversary of our initial public offering (or December 31, 2019); |
|
• |
the date on which we have, during the previous three-year period, issued more than $1 billion in non-convertible debt; or |
|
• |
the date on which we are deemed to be a “large accelerated filer” under the Securities Exchange Act of 1934, as amended, (i.e., the first day of the fiscal year after we have (i) more than $700 million in outstanding common equity held by our non-affiliates, measured each year on the last day of our second fiscal quarter, and (ii) been public for at least 12 months). |
The JOBS Act also provides that an emerging growth company can utilize the extended transition period provided in Section 7(a)(2)(B) of the Securities Act, for complying with new or revised accounting standards. We have elected to avail ourselves of this exemption and, as a result, our financial statements may not be comparable to the financial statements of issuers who are required to comply with the effective dates for new or revised accounting standards that are applicable to public companies. We cannot predict whether investors will find our common stock less attractive if we rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be reduced or more volatile.
For certain risks related to our status as an emerging growth company, see “Risk factors – Risks related to being a public company – We are an “emerging growth company” and the reduced disclosure requirements applicable to the emerging growth companies may make our common stock less attractive to investors.”
-5-
The offering
|
Common stock offered by the selling stockholders |
|
shares |
|
|
|
|
|
Common stock to be outstanding after this offering |
|
18,436,802 shares |
|
|
|
|
|
Underwriters’ option to purchase additional shares |
|
shares |
|
|
|
|
|
Use of proceeds |
|
We will not receive any of the proceeds from the sale of shares of common stock by the selling stockholders. |
|
|
|
|
|
Risk factors |
|
You should read the “Risk factors” section of this prospectus for a discussion of factors to consider carefully before deciding to invest in shares of our common stock. |
|
|
|
|
|
NASDAQ Global Select Market symbol |
|
“INGN” |
The number of shares of common stock to be outstanding following this offering is based on 18,436,802 shares of common stock outstanding as of September 30, 2014 and excludes:
|
• |
2,678,328 shares of common stock issuable upon the exercise of options to purchase common stock outstanding as of September 30, 2014, at a weighted average exercise price of $5.50 per share; |
|
• |
445,885 shares of common stock reserved for future grants under our stock-based compensation plans as September 30, 2014, consisting of: |
|
• |
297,174 shares of common stock reserved for future grants under our 2014 Equity Incentive Plan; and |
|
• |
148,711 shares of common stock reserved for future issuance under our 2014 Employee Stock Purchase Plan; |
|
• |
145,089 shares of common stock issuable upon the exercise of warrants outstanding as of September 30, 2014, at a weighted average exercise price of $0.30 per share; and |
|
• |
Any shares of common stock that become available subsequent to this offering under our 2014 Equity Incentive Plan and 2014 Employee Stock Purchase plan pursuant to provisions thereof that automatically increase the share reserves under such plans each year, as more fully described in “Employee benefit and stock plans.” |
Unless otherwise indicated, this prospectus reflects and assumes the following:
|
• |
No exercise by the underwriters of their option to purchase additional shares; and |
|
• |
No exercise of outstanding stock options or warrants subsequent to September 30, 2014. |
-6-
Summary financial data
We have derived the following summary of statements of operations data for the years ended December 31, 2013, 2012 and 2011 from audited financial statements incorporated by reference in this prospectus from our Annual Report on Form 10-K for the fiscal year ended December 31, 2013, or our 2013 Annual Report. We derived the following statements of operations data for the six months ended June 30, 2014 and 2013 and the balance sheet data as of June 30, 2014 from unaudited interim financial statements incorporated by reference in this prospectus from our Quarterly Report on Form 10-Q for the quarter ended June 30, 2014, or our June 2014 Quarterly Report. In the opinion of management, the unaudited financial statements reflect all adjustments, which include only normal recurring adjustments necessary for a fair statement of results of operations and financial position. You should read this data together with our financial statements and related notes, as well as the information under the captions “Selected financial data” herein and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our 2013 Annual Report and June 2014 Quarterly Report, which are incorporated by reference herein. Our historical results are not necessarily indicative of our future results, and results of interim periods are not necessarily indicative of results for the entire year.
|
|
Year ended December 31, |
|
|
Six months ended June 30, |
|
||||||||||||||
|
|
|
2013 |
|
|
|
2012 |
|
|
|
2011 |
|
|
|
2014 |
|
|
|
2013 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(unaudited) |
|
|||||
|
(amounts in thousands, except share and per share amounts) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenue |
$ |
75,443 |
|
|
$ |
48,576 |
|
|
$ |
30,634 |
|
|
$ |
54,026 |
|
|
$ |
35,904 |
|
|
Total cost of revenue |
|
36,452 |
|
|
|
24,627 |
|
|
|
15,930 |
|
|
|
26,974 |
|
|
|
16,730 |
|
|
Gross profit |
|
38,991 |
|
|
|
23,949 |
|
|
|
14,704 |
|
|
|
27,052 |
|
|
|
19,174 |
|
|
Operating expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
2,398 |
|
|
|
2,262 |
|
|
|
1,789 |
|
|
|
1,514 |
|
|
|
1,143 |
|
|
Sales and marketing |
|
18,375 |
|
|
|
12,569 |
|
|
|
9,014 |
|
|
|
12,069 |
|
|
|
8,742 |
|
|
General and administrative |
|
13,754 |
|
|
|
8,289 |
|
|
|
5,623 |
|
|
|
7,957 |
|
|
|
6,264 |
|
|
Total operating expenses |
|
34,527 |
|
|
|
23,120 |
|
|
|
16,426 |
|
|
|
21,540 |
|
|
|
16,149 |
|
|
Income (loss) from operations |
|
4,464 |
|
|
|
829 |
|
|
|
(1,722 |
) |
|
|
5,512 |
|
|
|
3,025 |
|
|
Total other expense, net |
|
(616 |
) |
|
|
(247 |
) |
|
|
(267 |
) |
|
|
(271 |
) |
|
|
(227 |
) |
|
Income (loss) before provision (benefit) for income taxes |
|
3,848 |
|
|
|
582 |
|
|
|
(1,989 |
) |
|
|
5,241 |
|
|
|
2,798 |
|
|
Provision (benefit) for income taxes |
|
(21,587 |
) |
|
|
18 |
|
|
|
13 |
|
|
|
2,067 |
|
|
|
108 |
|
|
Net income (loss) |
$ |
25,435 |
|
|
$ |
564 |
|
|
$ |
(2,002 |
) |
|
$ |
3,174 |
|
|
$ |
2,690 |
|
|
Less deemed dividend on redeemable convertible preferred stock |
|
(7,278 |
) |
|
|
(5,781 |
) |
|
|
(3,027 |
) |
|
|
(987 |
) |
|
|
(3,508 |
) |
|
Net income (loss) before preferred rights dividend |
$ |
18,157 |
|
|
$ |
(5,217 |
) |
|
$ |
(5,029 |
) |
|
$ |
2,187 |
|
|
$ |
(818 |
) |
|
Less preferred rights dividend on redeemable convertible preferred stock |
|
(7,165 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Less undistributed earnings to preferred stock |
|
(10,781 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Net income (loss) attributable to common stockholders |
$ |
211 |
|
|
$ |
(5,217 |
) |
|
$ |
(5,029 |
) |
|
$ |
2,187 |
|
|
$ |
(818 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic net income (loss) per share attributable to common stockholders |
$ |
0.76 |
|
|
$ |
(19.97 |
) |
|
$ |
(20.15 |
) |
|
$ |
0.13 |
|
|
$ |
(2.98 |
) |
|
Diluted net income (loss) per share attributable to common stockholders |
$ |
0.68 |
|
|
$ |
(19.97 |
) |
|
$ |
(20.15 |
) |
|
$ |
0.11 |
|
|
$ |
(2.98 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average number of shares used in calculating income (loss) per share attributable to common stockholders (note 1): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic common shares |
|
276,535 |
|
|
|
261,268 |
|
|
|
249,519 |
|
|
|
13,843,803 |
|
|
|
274,396 |
|
|
Diluted common shares |
|
2,008,156 |
|
|
|
261,268 |
|
|
|
249,519 |
|
|
|
15,826,754 |
|
|
|
274,396 |
|
|
(1) |
See note 2 to our 2013 Annual Report and our interim financial statements included in our June 2014 Quarterly Report for an explanation of the calculations of our basic and diluted net income (loss) per share attributable to common stockholders. |
|
|
|
As of December 31, |
|
|
As of June 30, |
|
||||||||||
|
(amounts in thousands) |
|
2013 |
|
|
2012 |
|
|
2011 |
|
|
2014 |
|
||||
|
|
|
|
|
|
(unaudited) |
|
||||||||||
|
Balance sheet data: |
|
|
|
|
|
|
|
|
|
|
||||||
|
Cash and cash equivalents |
|
$ |
13,521 |
|
|
$ |
15,112 |
|
|
$ |
3,906 |
|
|
$ |
69,046 |
|
|
Working capital |
|
|
13,159 |
|
|
|
12,880 |
|
|
|
1,302 |
|
|
|
71,394 |
|
|
Total assets |
|
|
82,397 |
|
|
|
47,586 |
|
|
|
24,131 |
|
|
|
145,466 |
|
|
Total indebtedness |
|
|
10,649 |
|
|
|
8,936 |
|
|
|
9,629 |
|
|
|
13,413 |
|
|
Deferred revenue |
|
|
2,263 |
|
|
|
1,094 |
|
|
|
594 |
|
|
|
3,530 |
|
|
Total liabilities |
|
|
26,098 |
|
|
|
19,011 |
|
|
|
16,575 |
|
|
|
34,652 |
|
|
Redeemable convertible preferred stock |
|
|
118,671 |
|
|
|
109,345 |
|
|
|
83,122 |
|
|
|
— |
|
|
Total stockholders’ (deficit) equity |
|
$ |
(62,372 |
) |
|
$ |
(80,770 |
) |
|
$ |
(75,566 |
) |
|
$ |
110,814 |
|
-7-
Non-GAAP financial measures
EBITDA, Adjusted EBITDA, and Adjusted net income (loss) are financial measures that are not calculated in accordance with generally accepted accounting principles in the United States, or GAAP. We define EBITDA as net income or loss excluding interest income, interest expense, taxes and depreciation and amortization. Adjusted EBITDA also excludes the change in the fair value of our preferred stock warrant liability and stock-based compensation. Below, we have provided a reconciliation of EBITDA, Adjusted EBITDA, and Adjusted net income (loss) to our net income or loss, the most directly comparable financial measure calculated and presented in accordance with GAAP. EBITDA, Adjusted EBITDA, and Adjusted net income (loss) should not be considered alternatives to net income or loss or any other measure of financial performance calculated and presented in accordance with GAAP. Our EBITDA, and Adjusted EBITDA, and Adjusted net income (loss) may not be comparable to similarly titled measures of other organizations because other organizations may not calculate EBITDA, Adjusted EBITDA, and Adjusted net income (loss) in the same manner as we calculate these measures.
We include EBITDA, Adjusted EBITDA and Adjusted net income (loss) in this prospectus because they are important measures upon which our management assesses our operating performance. We use EBITDA, Adjusted EBITDA and Adjusted net income (loss) as key performance measures because we believe they facilitate operating performance comparisons from period to period by excluding potential differences primarily caused by variations in capital structures, tax positions, the impact of depreciation and amortization expense on our fixed assets, changes related to the fair value re-measurements of our preferred stock warrant, and the impact of stock-based compensation expense. Because EBITDA, Adjusted EBITDA and Adjusted net income (loss) facilitate internal comparisons of our historical operating performance on a more consistent basis, we also use EBITDA, Adjusted EBITDA and Adjusted net income (loss) for business planning purposes, to incentivize and compensate our management personnel, and in evaluating acquisition opportunities. In addition, we believe EBITDA, Adjusted EBITDA and Adjusted net income (loss) and similar measures are widely used by investors, securities analysts, ratings agencies, and other parties in evaluating companies in our industry as a measure of financial performance and debt-service capabilities.
Our use of EBITDA, Adjusted EBITDA and Adjusted net income (loss) have limitations as analytical tools, and you should not consider them in isolation or as a substitute for analysis of our results as reported under GAAP. Some of these limitations are:
|
• |
EBITDA, Adjusted EBITDA and Adjusted net income (loss) do not reflect our cash expenditures for capital equipment or other contractual commitments; |
|
• |
Although depreciation and amortization are non-cash charges, the assets being depreciated and amortized may have to be replaced in the future, and EBITDA, Adjusted EBITDA and Adjusted net income (loss) do not reflect capital expenditure requirements for such replacements; |
|
• |
EBITDA, Adjusted EBITDA and Adjusted net income (loss) do not reflect changes in, or cash requirements for, our working capital needs; |
|
• |
EBITDA, Adjusted EBITDA, and Adjusted net income (loss) do not reflect the interest expense or the cash requirements necessary to service interest or principal payments on our indebtedness; and |
|
• |
Other companies, including companies in our industry, may calculate EBITDA, Adjusted EBITDA and Adjusted net income (loss) measures differently, which reduces their usefulness as a comparative measure. |
In evaluating EBITDA, Adjusted EBITDA, and Adjusted net income (loss) you should be aware that in the future we will incur expenses similar to the adjustments in this presentation. Our presentation of EBITDA, Adjusted EBITDA and Adjusted net income (loss) should not be construed as an inference that our future results will be unaffected by these expenses or any unusual or non-recurring items. When evaluating our performance, you should consider EBITDA, Adjusted EBITDA and Adjusted net income (loss) alongside other financial performance measures, including our net loss and other GAAP results.
-8-
The following table presents a reconciliation of EBITDA, Adjusted EBITDA and Adjusted net income (loss) to our net income or loss, the most comparable GAAP measure, for each of the periods indicated:
|
(amounts in thousands) |
|
Year ended December 31, |
|
|
Six months ended June 30, |
|
||||||||||||||
|
EBITDA (1) |
|
2013 |
|
|
2012 |
|
|
2011 |
|
|
2014 |
|
|
2013 |
|
|||||
|
Unaudited |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
25,435 |
|
|
$ |
564 |
|
|
$ |
(2,002 |
) |
|
$ |
3,174 |
|
|
$ |
2,690 |
|
|
Non-GAAP adjustments: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense |
|
|
562 |
|
|
|
493 |
|
|
|
261 |
|
|
|
336 |
|
|
|
199 |
|
|
Interest income |
|
|
(12 |
) |
|
|
(88 |
) |
|
|
(113 |
) |
|
|
(18 |
) |
|
|
(6 |
) |
|
Provision (benefit) for income taxes |
|
|
(21,587 |
) |
|
|
18 |
|
|
|
13 |
|
|
|
2,067 |
|
|
|
108 |
|
|
Depreciation and amortization |
|
|
8,544 |
|
|
|
4,984 |
|
|
|
3,198 |
|
|
|
5,586 |
|
|
|
3,653 |
|
|
EBITDA |
|
|
12,942 |
|
|
|
5,971 |
|
|
|
1,357 |
|
|
|
11,145 |
|
|
|
6,644 |
|
|
Change in fair value of preferred stock warrant liability |
|
|
262 |
|
|
|
(148 |
) |
|
|
119 |
|
|
|
(36 |
) |
|
|
243 |
|
|
Stock-based compensation |
|
|
230 |
|
|
|
60 |
|
|
|
144 |
|
|
|
666 |
|
|
|
51 |
|
|
Adjusted EBITDA |
|
$ |
13,434 |
|
|
$ |
5,883 |
|
|
$ |
1,620 |
|
|
$ |
11,775 |
|
|
$ |
6,938 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) (GAAP) |
|
$ |
25,435 |
|
|
$ |
564 |
|
|
$ |
(2,002 |
) |
|
$ |
3,174 |
|
|
$ |
2,690 |
|
|
One-time benefit from reversal of deferred tax valuation adjustment |
|
|
(21,807 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Adjusted net income (loss) |
|
$ |
3,628 |
|
|
$ |
564 |
|
|
$ |
(2,002 |
) |
|
$ |
3,174 |
|
|
$ |
2,690 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(amounts in thousands, except share and per share amounts) |
|
Year ended December 31, |
|
|
|
|
|
|
Six months ended June 30, |
|
||||||||||
|
Pro-forma non-GAAP results of EPS calculation (2)(3)(4) |
|
2013 |
|
|
2012 |
|
|
|
|
|
|
2014 |
|
|
2013 |
|
||||
|
Unaudited |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Numerator - basic and diluted: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income |
|
$ |
25,435 |
|
|
$ |
564 |
|
|
|
|
|
|
$ |
3,174 |
|
|
$ |
2,690 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pro-forma net income per share - basic common stock |
|
$ |
1.74 |
|
|
$ |
0.04 |
|
|
|
|
|
|
$ |
0.18 |
|
|
$ |
0.19 |
|
|
Pro-forma net income per share - diluted common stock |
|
$ |
1.55 |
|
|
$ |
0.04 |
|
|
|
|
|
|
$ |
0.16 |
|
|
$ |
0.17 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Denominator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pro-forma weighted-average common shares - basic common stock |
|
|
14,636,950 |
|
|
|
14,601,861 |
|
|
|
|
|
|
|
17,308,133 |
|
|
|
14,530,870 |
|
|
Pro-forma weighted-average common shares - diluted common stock |
|
|
16,368,571 |
|
|
|
15,486,487 |
|
|
|
|
|
|
|
19,291,084 |
|
|
|
15,654,526 |
|
|
(1) |
For a discussion of our use of EBITDA, Adjusted EBITDA, and Adjusted net income (loss) and their calculations, please see “—Non GAAP financial measures.” |
|
(2) |
The pro forma EPS calculations gives effect to: the automatic conversion of the outstanding convertible preferred stock into a weighted average of 14,219,001 and 14,216,838 shares of common stock, for the years ended December 31, 2013 and 2012, and 14, 219,001 and 14,057,509 for the six months ended June 30, 2014 and 2013. |
|
(3) |
The pro forma EPS calculations gives effect to: the cash exercise of warrants to purchase an aggregate of 142,495 shares of common stock, which was expected to occur prior to closing of the initial public offering as the warrants would have otherwise expired at that time for the years ended December 31, 2013 and 2012 and 130,385 for the six months ended June 30, 2013. |
|
(4) |
The pro forma EPS calculations give effect to the reclassification of our preferred stock warrant liability to additional paid-in-capital upon the closing of our initial public offering. |
-9-
Investing in our common stock involves a high degree of risk. You should consider carefully the risks and uncertainties described below, together with all of the other information in this prospectus or incorporated by reference into this prospectus from our 2013 Annual Report, our June 2014 Quarterly Report and our other filings with the Securities Exchange Commission listed in the section of the prospectus entitled “Incorporation by reference,” before deciding whether to purchase shares of our common stock. If any of the following risks are realized, our business, financial condition, results of operations and prospects could be materially and adversely affected. In that event, the price of our common stock could decline and you could lose part or all of your investment.
Risks related to our business and strategy
A significant majority of our customers have health coverage under the Medicare program, and recently enacted and future changes in the reimbursement rates or payment methodologies under Medicare and other government programs have affected and could continue to materially and adversely affect our business and operating results.
As a provider of oxygen product rentals, we have historically depended heavily on Medicare reimbursement as a result of the higher proportion of elderly persons suffering from chronic respiratory conditions. Medicare Part B, or Supplementary Medical Insurance Benefits, provides coverage to eligible beneficiaries that include items of durable medical equipment for use in the home, such as oxygen equipment and other respiratory devices. We believe that more than 60% of oxygen therapy patients in the United States have primary coverage under Medicare Part B. For the six months ended June 30, 2014 and June 30, 2013, we derived approximately 25.0% and 29.5%, respectively, of our total revenue from the Medicare’s program or beneficiaries (including patient co-insurance obligations). There are increasing pressures on Medicare to control healthcare costs and to reduce or limit reimbursement rates for home medical products.
Legislation, including the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, the Deficit Reduction Act of 2005, the Medicare Improvements for Patients and Providers Act of 2008, and the Patient Protection and Affordable Care Act, contain provisions that directly impact reimbursement for the durable medical equipment products provided by us:
|
• |
The Medicare Prescription Drug, Improvement, and Modernization Act of 2003 significantly reduced reimbursement for inhalation drug therapies beginning in 2005, reduced payment amounts for certain durable medical equipment, including oxygen, beginning in 2005, froze payment amounts for other covered home medical equipment items through 2008, established a competitive bidding program for home medical equipment and implemented quality standards and accreditation requirements for durable medical equipment suppliers. |
|
• |
The Deficit Reduction Act of 2005 limited the total number of continuous rental months for which Medicare will pay for oxygen equipment to 36 months, after which time there is generally no additional reimbursement to the supplier (other than for periodic, in-home maintenance and servicing). The Deficit Reduction Act of 2005 also provided that title of the equipment would transfer to the beneficiary, which was later repealed by the Medicare Improvements for Patients and Providers Act of 2008. For purposes of the rental cap, the Deficit Reduction Act of 2005 provided for a new 36-month rental period that began January 1, 2006 for all oxygen equipment. After the 36th continuous month during which payment is made for the oxygen equipment, the supplier is generally required to continue to furnish the equipment during the period of medical need for the remainder of the useful lifetime of the equipment, provided there are no breaks in service due to medical necessity that exceed 60 days. The reasonable useful lifetime for portable oxygen equipment is 60 months. After 60 months, if the patient requests, the rental cycle starts over and a new 36-month capped rental period begins. There are no limits on the number of 60-month cycles over which a Medicare patient may receive benefits and an oxygen therapy provider may receive reimbursement, so long as such equipment continues to be medically necessary for the patient. We anticipate that the Deficit Reduction Act of 2005 oxygen payment rules will continue to negatively affect our net revenue on an ongoing basis, as each month additional customers reach the 36-month capped service period, resulting in potentially two or more years without rental income from these customers. We cannot state with certainty the number of patients in the capped rental period or the potential impact to revenue associated with patients in the capped rental period. |
|
• |
The Medicare Improvements for Patients and Providers Act of 2008 retroactively delayed the implementation of competitive bidding for 18 months from previously established dates and decreased the 2009 fee schedule payment amounts by 9.5% for product categories included in competitive bidding. In addition to the 9.5% reduction under Medicare Improvements for Patients and Providers Act of 2008, the Centers for Medicare & Medicaid Services implemented a reduction to the monthly payment amount for stationary oxygen equipment. The monthly payment rate for non-delivery ambulatory oxygen in the relevant period was flat at $51.63. The table below summarizes the increases and decreases in the monthly payment amounts for stationary oxygen equipment. |
|
(dollars in hundreds) |
|
2009 |
|
|
2010 |
|
|
2011 |
|
|
2012 |
|
|
2013 |
|
|
2014 |
|
||||||
|
Stationary oxygen percentage rate changes |
|
|
-2.30 |
% |
|
|
-1.50 |
% |
|
|
0.10 |
% |
|
|
1.60 |
% |
|
|
0.70 |
% |
|
|
0.50 |
% |
|
Stationary oxygen monthly payment amounts |
|
$ |
175.79 |
|
|
$ |
173.17 |
|
|
$ |
173.31 |
|
|
$ |
176.06 |
|
|
$ |
177.36 |
|
|
$ |
178.24 |
|
-10-
|
• |
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, includes, among other things, a deductible excise tax on any entity that manufactures or imports medical devices offered for sale in the United States, with limited exceptions including oxygen products such as ours, which began in 2013, new face-to-face physician encounter requirements for durable medical equipment and home health services, and a requirement that by 2016, the competitive bidding process must be nationalized or prices in non-competitive bidding areas must be adjusted to match competitive bidding prices. |
These legislative provisions, as currently in effect and when fully implemented, have had and will continue to have a material and adverse effect on our business, financial condition, and operating results.
Due to budgetary shortfalls, many states are considering, or have enacted, cuts to their Medicaid programs. These cuts have included, or may include, elimination or reduction of coverage for our products, amounts eligible for payment under co-insurance arrangements, or payment rates for covered items. Continued state budgetary pressures could lead to further reductions in funding for the reimbursement for our products which, in turn, would adversely affect our business, financial conditions, and results of operations.
The implementation of the competitive bidding process under Medicare could negatively affect our business and financial condition.
The Medicare Prescription Drug, Improvement, and Modernization Act of 2003 required the Secretary of Health and Human Services to establish and implement programs under which competitive acquisition areas are established throughout the United States for purposes of awarding contracts for the furnishing of competitively priced items of durable medical equipment, including oxygen equipment.
The Centers for Medicare & Medicaid Services, the agency responsible for administering the Medicare program, conducts a competition for each competitive acquisition area under which providers submit bids to supply certain covered items of durable medical equipment. Successful bidders must meet certain program quality standards in order to be awarded a contract and only successful bidders can supply the covered items to Medicare beneficiaries in the acquisition area. There are, however, regulations in place that allow non-contracted providers to continue to provide products and services to their existing customers at the new competitive bidding payment amounts. The contracts are expected to be re-bid every three years. The Centers for Medicare & Medicaid Services is required to award contracts to multiple entities submitting bids in each area for an item or service, but has the authority to limit the number of contractors in a competitive acquisition area to the number it determines to be necessary to meet projected demand.
Although the Centers for Medicare & Medicaid Services concluded the bidding process for the first round of Metropolitan Statistical Areas in September 2007, in July 2008, Congress enacted the Medicare Improvements for Patients and Providers Act of 2008, which retroactively delayed the implementation of competitive bidding. The Medicare Improvements for Patients and Providers Act of 2008 also reduced Medicare prices nationwide by 9.5% beginning in 2009 for the product categories, including oxygen, that were initially included in competitive bidding.
In 2009, the Centers for Medicare & Medicaid Services implemented a new bidding process in nine Metropolitan Statistical Areas, covering approximately 9% of the Medicare oxygen market. Reimbursement rates from the re-bidding process were publicly released by the Centers for Medicare & Medicaid Services on June 30, 2010. The Centers for Medicare & Medicaid Services announced average savings of approximately 35% off the current standard Medicare payment rates in effect for the product categories included in competitive bidding. As of January 1, 2011, these payment rates were in effect in the nine markets only. We were offered six three-year contracts to provide oxygen equipment in six of the nine markets, and we accepted and signed those contracts.
The Centers for Medicare & Medicaid Services implemented the second phase of competitive bidding in an additional 100 competitive bidding areas covering approximately 50% of the Medicare oxygen market, with three-year contracts effective July 1, 2013. The Centers for Medicare & Medicaid Services announced average savings of approximately 45% off the current standard Medicare payment rates in effect for the product categories included in competitive bidding. As of July 1, 2013, these payment rates were in effect in the 100 competitive bidding areas. We were offered 89 contracts to provide oxygen equipment in 89 of the 100 competitive bidding areas, and we accepted and signed those contracts.
Round one re-compete rates went into effect on January 1, 2014; reimbursement rates from the re-bidding process were publicly released by the Centers for Medicare & Medicaid Services on October 1, 2013. The Centers for Medicare & Medicaid Services announced average savings of approximately 37% off the current standard Medicare payment rates in effect from the product categories included in competitive bidding. We were offered 3 contracts to provide respiratory equipment in 3 of the 9 competitive bidding areas, and we accepted and signed those contracts. We are required to be able to supply additional respiratory products such as sleep and aerosol therapy, which have lower margins than our existing products. This could have a negative impact on our financial conditions and results of operations.
-11-
The Patient Protection and Affordable Care Act legislation requires the Centers for Medicare & Medicaid Services to expand competitive bidding further to additional geographic markets or to use competitive bid pricing information to adjust the payment amounts otherwise in effect for areas that are not competitive acquisition areas by January 1, 2016.
In July 2014, the Centers for Medicare and Medicaid services released a proposed rule that sets forth methodologies to adjust the fee schedule amounts for items subject to competitive bidding in areas where competitive bidding are not implemented. The proposed rule applies rate reductions to all un-bid areas instead of doing an additional bidding process. The fee schedules in the un-bid areas would be adjusted based on regional averages of the single payment amounts for areas already under competitive bidding. The regional prices would be limited by a national ceiling (110% of the average of the regional prices) and a floor (90% of the average regional prices). The regions would be defined as follows:
|
Region Name |
|
States Covered |
|
Far West |
|
CA, NV, OR, WA |
|
Great Lakes |
|
IL, IN, MI, OH, WI |
|
Mideast |
|
DC, DE, MD, NJ, NY, PA |
|
New England |
|
CT, MA, NH, RI |
|
Plains |
|
IA, KS, MN, MO, NE |
|
Rocky Mountain |
|
CO, ID, UT |
|
Southeast |
|
AL, AR, FL, GA, KY, LA, NC, SC, TN, VA |
|
Southwest |
|
AZ, NM, OK, TX |
The Centers for Medicare and Medicaid proposes to define frontier states as states where more than 50% of the counties in the state have a population density of 6 people or less per square mile and rural states are defined as states where more than 50% of the population lives in rural areas per census data. Current frontier states include MT, ND, SD and WY; rural states include ME, MS, VT and WV; and non-contiguous United States areas include AK, HI, Guam and Puerto Rico. For frontier and rural states, the single payment amount would be the national ceiling (110% of the average of the regional prices) to account for higher servicing costs in these areas. For non-contiguous United States areas, single payment amounts would be the higher of the national ceiling, or the average of competitive bidding pricing from these areas, if the areas had been bid through competitive bidding.
In addition, the Centers for Medicare and Medicaid Services is proposing to phase in bundled monthly payments for oxygen in no more than 12 CBAs, removing the 36 month cap on oxygen equipment payments, removing the separate add-on payments for non-delivery portable equipment and removing the separate payments for oxygen content. The payment would be bundled under one monthly rental fee for all rented equipment and related accessories, maintenance and servicing of the equipment and delivery of oxygen contents, and pricing would be determined through an additional competitive bidding round. The Centers for Medicare and Medicaid Services noted in the proposed rule that the 36-month cap is difficult to administer and few patients receive benefits in the capped period, noting that only 25% of the patients reach the end of the 36 month period. In this proposal, the current premium reimbursement for oxygen generating portable equipment, including our portable oxygen concentrators, would be eliminated. We cannot predict the impact this proposed rule, if finalized, would have on our business, financial conditions and results of operations.
The Centers for Medicare and Medicaid Services also announced in July 2014 the schedule for competitive bidding round two re-compete, associated with approximately 50% of the market with contracts set to expire June 30, 2016. The Centers for Medicare and Medicaid Services intends to announce the bidding schedule in fall 2014 and commence bidding in winter 2015. The Centers for Medicare and Medicaid Services updated the product categories and the competitive bidding areas. Respiratory equipment includes oxygen, oxygen equipment, continuous positive airway pressure devices, respiratory assist devices and related supplies and accessories, such that nebulizers, which are now their own separate product category instead of being included in the respiratory equipment category. Round two re-compete is in the same geographic areas that were included in the original round two. However, as a result of the Office of Management and Budget’s updates to the original 91 round two metropolitan statistical areas, there are now 90 metropolitan statistical areas for round two re-compete. The round two re-compete competitive bidding areas have nearly the same ZIP codes as the round two competitive bidding areas, the associated changes in the zip codes since the competitive bidding was implemented are reflective in this round two re-compete. Also, competitive bidding areas that were located in multi-state metropolitan statistical areas were defined so that no competitive bidding area is included in more than one state.
While we are monitoring the development and implementation of this proposal, we believe that the net effect of the proposal would be an approximately 4-5% decrease in 2016 revenue should the proposal become effective in its current form. Additionally, we expect that the stationary oxygen and non-delivery ambulatory oxygen payment rates will continue to fluctuate and a large negative payment adjustment could adversely affect our business, financial conditions and results of operations.
Although we continue to monitor developments regarding the implementation of the competitive bidding program, we cannot predict the outcome of the competitive bidding program on our business when fully implemented, nor the Medicare payment rates that will be
-12-
in effect in future years for the items subject to competitive bidding, including our products. We expect that the stationary oxygen and non-delivery ambulatory oxygen payment rates will continue to fluctuate, and a large negative payment adjustment could adversely affect our business, financial conditions and results of operations.
We face intense national, regional and local competition and if we are unable to compete successfully, it could have an adverse effect on our revenue, revenue growth rate, if any, and market share.
The oxygen therapy market is a highly competitive industry. We compete with a number of manufacturers and distributors of portable oxygen concentrators, as well as providers of other oxygen therapy solutions such as home delivery of oxygen tanks or cylinders.
Our significant manufacturing competitors are Invacare Corporation, Respironics (a subsidiary of Koninklijke Philips N.V.), AirSep Corporation and SeQual Technologies (subsidiaries of Chart Industries, Inc.), Inova Labs, Inc. and DeVilbiss Healthcare. Given the relatively straightforward regulatory path in the oxygen therapy device manufacturing market, we expect that the industry will become increasingly competitive in the future. Manufacturing companies compete for sales to providers primarily on the basis of product features, service and price.
For many years, Lincare Inc., Apria Healthcare, Inc. Rotech Healthcare, Inc. and American HomePatient, Inc. have been among the market leaders in providing oxygen therapy, while the remaining oxygen therapy market is serviced by local providers. Because many oxygen therapy providers were either excluded from contracts in the Medicare competitive bidding process, or will have difficulty providing service at the prevailing Medicare reimbursement rates, we expect more industry consolidation. Oxygen therapy providers compete primarily on the basis of product features and service, rather than price, since reimbursement levels are established by Medicare and Medicaid, or by the individual determinations of private payors.
Some of our competitors are large, well-capitalized companies with greater resources than we have. As a consequence, they are able to spend more aggressively on product development, marketing, sales and other product initiatives than we can. Some of these competitors have:
|
• |
significantly greater name recognition; |
|
• |
established relations with healthcare professionals, customers and third-party payors; |
|
• |
established distribution networks; |
|
• |
additional lines of products, and the ability to offer rebates or bundle products to offer higher discounts or other incentives to gain a competitive advantage; |
|
• |
greater history in conducting research and development, manufacturing, marketing and obtaining regulatory approval for oxygen device products; and |
|
• |
greater financial and human resources for product development, sales and marketing, patent litigation and customer financing. |
As a result, our competitors may be able to respond more quickly and effectively than we can to new or changing opportunities, technologies, standard regulatory and reimbursement development and customer requirements. In light of these advantages that our competitors maintain, even if our technology and direct-to-consumer distribution strategy is more effective than the technology and distribution strategy of our competitors, current or potential customers might accept competitor products and services in lieu of purchasing our products. We anticipate that we will face increased competition in the future as existing companies and competitors develop new or improved products and distribution strategies and as new companies enter the market with new technologies and distribution strategies. We may not be able to compete effectively against these organizations. Our ability to compete successfully and to increase our market share is dependent upon our reputation for providing responsive, professional and high-quality products and services and achieving strong customer satisfaction. Increased competition in the future could adversely affect our revenue, revenue growth rate, margins and market share.
Healthcare reform measures may have a material adverse effect on our business and results of operations.
In the United States, the legislative landscape, particularly as it relates to healthcare regulation and reimbursement coverage, continues to evolve. In March 2010, the Patient Protection and Affordable Care Act was passed, which has the potential to substantially change healthcare financing by both governmental and private insurers, and significantly impact the U.S. medical device industry. As discussed above, the Patient Protection and Affordable Care Act, among other things, imposes a new excise tax, which began in 2013, on entities that manufacture, produce or import medical devices in an amount equal to 2.3% of the price for which such devices are sold in the United States, however, oxygen products such as ours were exempt. In addition, as discussed above, the Patient Protection and Affordable Care Act also expands the round two of competitive bidding to a total of 91 competitive bidding areas, and by 2016, the process must be nationalized or prices in non-competitive bidding areas must be adjusted to match competitive bidding prices.
-13-
In addition, other legislative changes have been proposed and adopted in the United States since the Patient Protection and Affordable Care Act was enacted. On August 2, 2011, the Budget Control Act of 2011 among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions of Medicare payments to providers up to 2% per fiscal year, which went into effect on April 1, 2013, and will remain in effect through 2024 unless additional Congressional action is taken. On January 2, 2013, President Obama signed into law the American Taxpayer Relief Act of 2012 which, among other things, further reduced Medicare payments to certain providers, including physicians, hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our products or additional pricing pressures.
If we are unable to continue to enhance our existing products and develop and market new products that respond to customer needs and preferences and achieve market acceptance, we may experience a decrease in demand for our products and our business could suffer.
We may not be able to compete as effectively with our competitors, and ultimately satisfy the needs and preferences of our customers, unless we can continue to enhance existing products and develop new innovative products. Product development requires significant financial, technological, and other resources. While we expended $1.5 million and $1.1 million for research and development efforts for six months ended June 30, 2014 and June 30, 2013, respectively, we cannot assure you that this level of investment in research and development will be sufficient to maintain a competitive advantage in product innovation, which could cause our business to suffer. Product improvements and new product introductions also require significant planning, design, development, and testing at the technological, product, and manufacturing process levels and we may not be able to timely develop product improvements or new products. Our competitors’ new products may enter the market before our new products reach market, be more effective with more features, obtain better market acceptance, or render our products obsolete. Any new products that we develop may not receive market acceptance or otherwise generate any meaningful sales or profits for us relative to our expectations based on, among other things, existing and anticipated investments in manufacturing capacity and commitments to fund advertising, marketing, promotional programs, and research and development.
We depend upon reimbursement from Medicare, private payors and Medicaid for a significant portion of our revenue, and if we fail to manage the complex and lengthy reimbursement process, our business and operating results could suffer.
A significant portion of our revenue is derived from reimbursement by third-party payors. We accept assignment of insurance benefits from customers and, in a majority of cases, invoice and collect payments directly from Medicare, private payors and Medicaid, as well as from customers under co-insurance provisions. For the six months ended June 30, 2014 and June 30, 2013, approximately 34.6% and 39.7% of our total revenue was derived from Medicare, private payors, Medicaid, and individual customers who directly receive reimbursement from third-party payors.
Our financial condition and results of operations may be affected by the healthcare industry’s reimbursement process, which is complex and can involve lengthy delays between the time that a product is delivered to the consumer and the time that the reimbursement amounts are settled. Depending on the payor, we may be required to obtain certain payor-specific documentation from physicians and other healthcare providers before submitting claims for reimbursement. Certain payors have filing deadlines and they will not pay claims submitted after such time. We are also subject to extensive pre-payment and post-payment audits by governmental and private payors that could result in material delays, refunds of monies received or denials of claims submitted for payment under such third-party payor programs and contracts. We cannot ensure that we will be able to continue to effectively manage the reimbursement process and collect payments for our products promptly. If we fail to manage the complex and lengthy reimbursement process, it would adversely affect our business, financial conditions, and results of operations.
Failure to obtain private payor contracts and future reductions in reimbursement rates from private payors could have a material adverse effect on our financial condition and operating results.
A portion of our revenue is derived from private payors. Based on our patient population, we estimate at least 30% of potential customers have non-Medicare insurance coverage, and we believe these patients represent a younger and more active patient population that will be drawn to the quality-of-life benefits of our solution. Failing to maintain and obtain private payor contracts from private insurance companies and employers and secure in-network provider status could have a material adverse effect on our financial condition and operating results. In addition, private payors are under pressure to increase profitability and reduce costs. In response, certain private payors are limiting coverage or reducing reimbursement rates for the products we provide. We believe that private payor reimbursement levels will generally be reset in accordance with the Medicare payment amounts determined by competitive bidding. We cannot predict the extent to which reimbursement for our products will be affected by competitive bidding or
-14-
by initiatives to reduce costs for private payors. Failure to obtain or maintain private payor contracts or the unavailability of third-party coverage or inadequacy of reimbursement for our products would adversely affect our business, financial conditions, and results of operations.
We obtain some of the components, subassemblies and completed products included in our Inogen One systems from a single source or a limited group of manufacturers or suppliers, and the partial or complete loss of one of these manufacturers or suppliers could cause significant production delays, an inability to meet customer demand and a substantial loss in revenue.
We utilize single source suppliers for some of the components and subassemblies we use in our Inogen One systems. We have qualified alternate sources of supply sufficient to support future needs and we have taken other mitigating steps to reduce the impact of a change in supplier; however, there may be delays in switching to these alternative suppliers if our primary source is terminated without notice. Our dependence on single source suppliers of components may expose us to several risks, including, among other things:
|
• |
Our suppliers may encounter financial hardships as a result of unfavorable economic and market conditions unrelated to our demand for components, which could inhibit their ability to fulfill our orders and meet our requirements; |
|
• |
Suppliers may fail to comply with regulatory requirements, be subject to lengthy compliance, validation or qualification periods, or make errors in manufacturing components that could negatively affect the efficacy or safety of our products or cause delays in supplying of our products to our customers; |
|
• |
Newly identified suppliers may not qualify under the stringent regulatory standards to which our business is subject; |
|
• |
We or our suppliers may not be able to respond to unanticipated changes in customer orders, and if orders do not match forecasts, we or our suppliers may have excess or inadequate inventory of materials and components; |
|
• |
We may be subject to price fluctuations due to a lack of long-term supply arrangements for key components; |
|
• |
We may experience delays in delivery by our suppliers due to changes in demand from us or their other customers; |
|
• |
We or our suppliers may lose access to critical services and components, resulting in an interruption in the manufacture, assembly and shipment of our systems; |
|
• |
Our suppliers may be subject to allegations by other parties of misappropriation of proprietary information in connection with their supply of products to us, which could inhibit their ability to fulfill our orders and meet our requirements; |
|
• |
Fluctuations in demand for products that our suppliers manufacture for others may affect their ability or willingness to deliver components to us in a timely manner; |
|
• |
Our suppliers may wish to discontinue supplying components or services to us; and |
|
• |
We may not be able to find new or alternative components or reconfigure our system and manufacturing processes in a timely manner if the necessary components become unavailable. |
In addition, we may be deemed to manufacture or contract to manufacture products that contain certain minerals that have been designated as “conflict minerals” under the Dodd-Frank Wall Street Reform and Consumer Protection Act. As a result, in future periods, we may be required to perform due diligence to determine the origin of such minerals, and disclose and report whether or not such minerals originated in the Democratic Republic of the Congo or adjoining countries. The implementation of these new requirements could adversely affect the sourcing, availability, and pricing of minerals used in the manufacture of our products. In addition, we may incur additional costs to comply with the disclosure requirements, including costs related to determining the source of any of the relevant minerals and metals used in our products.
If any of these risks materialize, costs could significantly increase and our ability to meet demand for our products could be impacted. If we are unable to satisfy commercial demand for our Inogen One systems in a timely manner, our ability to generate revenue would be impaired, market acceptance of our products could be adversely affected, and customers may instead purchase or use alternative products. In addition, we could be forced to secure new or alternative components and subassemblies through a replacement supplier. Finding alternative sources for these components and subassemblies could be difficult in certain cases and may entail a significant amount of time and disruption. In some cases, we would need to change the components or subassemblies if we sourced them from an alternative supplier. This, in turn, could require a redesign of our Inogen One systems and, potentially, require additional FDA clearance or approval before we could use any redesigned product with new components or subassemblies, thereby causing further costs and delays that could adversely affect our business, financial condition and operating results.
We do not have long-term supply contracts with many of our third-party suppliers.
We purchase components and subassemblies from third-party suppliers, including some of our single source suppliers, through purchase orders and do not have long-term supply contracts with many of these third-party suppliers. Many of our third-party
-15-
suppliers, therefore, are not obligated to perform services or supply products to us for any specific period, in any specific quantity or at any specific price, except as may be provided in a particular purchase order. We do not maintain large volumes of inventory from most of these suppliers. If we inaccurately forecast demand for components or subassemblies, our ability to manufacture and commercialize our Inogen One systems could be delayed and our competitive position and reputation could be harmed. In addition, if we fail to effectively manage our relationships with these suppliers, we may be required to change suppliers which would be time consuming and disruptive and could adversely affect our business, financial condition and operating results.
If we fail to comply with U.S. export control and economic sanctions or fail to expand and maintain an effective sales force or successfully develop our international distribution network, our business, financial condition and operating results may be adversely affected.
We currently derive the majority of our revenue from rentals or sales generated from our own direct sales force. Failure to maintain or expand our direct sales force could adversely impact our financial and operating performance. Additionally, we use international distributors to augment our sales efforts, certain of which are exclusive distributors in certain foreign countries. We cannot assure you that we will be able to successfully develop our relationships with third-party distributors internationally. In addition, we are subject to United States export control and economic sanctions laws relating to the sale of our products, the violation of which could result in substantial penalties being imposed against us. In particular, we have secured annual export licenses from the U.S. Treasury Department’s Office of Foreign Assets Control to sell our products to a distributor and hospital and clinic end-users in Iran. The use of this license requires us to observe strict conditions with respect to products sold, end-user limitations and payment requirements. Although we believe we have maintained compliance with license requirements, there can be no assurance that the license will not be revoked, be renewed in the future or that we will remain in compliance. More broadly, if we fail to comply with export control laws or successfully develop our relationship with international distributors, our sales could fail to grow or could decline, and our ability to grow our business could be adversely affected. Distributors that are in the business of selling other medical products may not devote a sufficient level of resources and support required to generate awareness of our products and grow or maintain product sales. If our distributors are unwilling or unable to market and sell our products, or if they do not perform to our expectations, we could experience delayed or reduced market acceptance and sales of our products.
We may be subject to substantial warranty or product liability claims or other litigation in the ordinary course of business that may adversely affect our business, financial condition and operating results.
As manufacturers of medical devices, we may be subject to substantial warranty or product liability claims or other litigation in the ordinary course of business that may require us to make significant expenditures to defend these claims or pay damage awards. For example, our Inogen One systems contain lithium ion batteries, which, under certain circumstances, can be a fire hazard. We, as well as our key suppliers, maintain product liability insurance, but this insurance is limited in amount and subject to significant deductibles. There is no guarantee that insurance will be available or adequate to protect against all claims. Our insurance policies are subject to annual renewal and we may not be able to obtain liability insurance in the future on acceptable terms or at all. In addition, our insurance premiums could be subject to increases in the future, which may be material. If the coverage limits are inadequate to cover our liabilities or our insurance costs continue to increase as a result of warranty or product liability claims or other litigation, then our business, financial condition and operating results may be adversely affected.
Increases in our operating costs could have a material adverse effect on our business, financial condition and operating results.
Reimbursement rates are established by fee schedules mandated by Medicare, private payors and Medicaid, and are likely to remain constant or decrease due, in part, to federal and state government budgetary constraints. As a result, with respect to Medicare and Medicaid related revenue, we are not able to offset the effects of general inflation on our operating costs through increases in prices for our products. In particular, labor and related costs account for a significant portion of our operating costs and we compete with other healthcare providers to attract and retain qualified or skilled personnel and with various industries for administrative and service employees. This competitive environment could result in increased labor costs. As such, we must control our operating costs, particularly labor and related costs, and failing to do so could adversely affect our financial conditions and results of operations.
We depend on the services of our senior executives and other key technical personnel, the loss of whom could negatively affect our business.
Our success depends upon the skills, experience and efforts of our senior executives and other key technical personnel, including certain members of our engineering staff, and our sales and marketing executives. Much of our corporate expertise is concentrated in relatively few employees, the loss of which for any reason could negatively affect our business. Competition for our highly skilled employees is intense and we cannot prevent the resignation of any employee. We do not maintain “key man” life insurance on any of our senior executives. None of our senior executive team is bound by written employment contracts to remain with us for a specified period. In addition, we have not entered into non-compete agreements with members of our senior management team. The loss of any member of our senior management team could harm our ability to implement our business strategy and respond to the market conditions in which we operate.
-16-
We rely on information technology, and if we are unable to protect against service interruptions, data corruption, cyber-based attacks or network security breaches, our operations could be disrupted and our business could be negatively affected.
We rely on information technology networks and systems to process, transmit and store electronic and financial information; to coordinate our business; and to communicate within our company and with customers, suppliers, partners and other third-parties. These information technology systems may be susceptible to damage, disruptions or shutdowns, hardware or software failures, power outages, computer viruses, cyber attacks, telecommunication failures, user errors or catastrophic events. If our information technology systems suffer severe damage, disruption or shutdown, and our business continuity plans do not effectively resolve the issues in a timely manner, our operations could be disrupted and our business could be negatively affected. In addition, cyber attacks could lead to potential unauthorized access and disclosure of confidential information, and data loss and corruption. There is no assurance that we will not experience these service interruptions or cyber attacks in the future.
We incurred losses from inception until fiscal year 2012, and we have only recently achieved profitability.
We have a limited operating history and incurred significant net losses in each fiscal year until fiscal year 2012, when we achieved positive net income. As of June 30, 2014, we had an accumulated deficit of $60.3 million. These net losses have resulted principally from costs incurred in our research and development programs and from our selling, general and administrative expenses. We expect to incur increases in expenses for research and development and significant expansion of our sales and marketing capabilities. Additionally, since completing our initial public offering, we expect that our selling, general and administrative expenses will increase due to the additional operational and reporting costs associated with being a public company. Because of the numerous risks and uncertainties associated with our commercialization efforts and future product development, we are unable to predict if we will maintain or increase our net income.
Our financial results may vary significantly from quarter-to-quarter due to a number of factors, which may lead to volatility in our stock price.
Our quarterly revenue and results of operations have varied in the past and may continue to vary significantly from quarter-to-quarter. This variability may lead to volatility in our stock price as research analysts and investors respond to these quarterly fluctuations. These fluctuations are due to numerous factors, including: fluctuations in consumer demand for our products; seasonal cycles in consumer spending; our ability to design, manufacture and deliver products to our consumers in a timely and cost-effective manner; quality control problems in our manufacturing operations; our ability to timely obtain adequate quantities of the components used in our products; new product introductions and enhancements by us and our competitors; unanticipated increases in costs or expenses; and fluctuations in foreign currency exchange rates. For example, we typically experience higher sales in the second quarter, as a result of consumers traveling and vacationing during the summer months. The foregoing factors are difficult to forecast, and these, as well as other factors, could materially and adversely affect our quarterly and annual results of operations. In addition, a significant amount of our operating expenses are relatively fixed due to our manufacturing, research and development, and sales and general administrative efforts. Any failure to adjust spending quickly enough to compensate for a revenue shortfall could magnify the adverse impact of such revenue shortfall on our results of operations. Our results of operations may not meet the expectations of research analysts or investors, in which case the price of our common stock could decrease significantly.
An adverse outcome of a sales and use tax audit could have a material adverse effect on our results of operations and financial condition.
The California State Board of Equalization conducted a sales and use tax audit of our operations in California in 2008. As a result of the audit, the California State Board of Equalization confirmed that our sales are not subject to California sales and use tax. We believe that our sales in other states should not be subject to sales and use tax. There can be no assurance, however, that other states may agree with our position and we may be subject to an audit that may not be resolved in our favor. Such an audit could be expensive and time-consuming and result in substantial management distraction. If the matter were to be resolved in a manner adverse to us, it could have a material adverse effect on our results of operations and financial position.
Our ability to use net operating losses to offset future taxable income may be subject to certain limitations.
As of December 31, 2013, we had federal and state net operating loss carryforwards, or NOLs, of approximately $56.7 million and $54.9 million, respectively. They expire in various years beginning in 2022 and 2013, respectively, if not utilized. In general, under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, a corporation that undergoes an “ownership change” is subject to limitations on its ability to utilize its pre-change NOLs to offset future taxable income. In general, an “ownership change” occurs if there is a cumulative change in our ownership by “5% shareholders” that exceeds 50 percentage points over a rolling three-year period. Our existing NOLs may be subject to limitations arising from previous ownership changes, and if we undergo one or more ownership changes in connection with this offering or future transactions in our stock, our ability to utilize NOLs could be further limited by Section 382 of the Code. Even after factoring in these limitations, we were able to determine based on future
-17-
projections of income that it is more likely than not that all of our federal NOLs will be utilized before they expire and therefore determined that releasing the valuation allowance relating to these NOLs was appropriate during this period. However, we determined that some of our California NOLs will expire unused and therefore we have maintained a valuation allowance of $4.1 million relating to these NOLs.
Risks related to the regulatory environment
We are subject to extensive Federal and state regulation, and if we fail to comply with applicable regulations, we could suffer severe criminal or civil sanctions or be required to make significant changes to our operations that could adversely affect our business, financial condition and operating results.
The federal government and all states in which we currently operate regulate various aspects of our business. In particular, our sales and customer service centers are subject to federal laws that regulate interstate motor-carrier transportation. Our operations also are subject to state laws governing, among other things, distribution of medical equipment and certain types of home health activities, and we are required to obtain and maintain licenses in each state to act as a durable medical equipment supplier. Certain of our employees are subject to state laws and regulations governing the professional practices of respiratory therapy.
As a healthcare provider participating in governmental healthcare programs, we are subject to laws directed at preventing fraud and abuse, which subject our marketing, billing, documentation and other practices to government scrutiny. To ensure compliance with Medicare, Medicaid and other regulations, government agencies or their contractors often conduct routine audits and request customer records and other documents to support our claims submitted for payment of services rendered. Government agencies or their contractors also periodically open investigations and obtain information from healthcare providers. Violations of federal and state regulations can result in severe criminal, civil and administrative penalties and sanctions, including debarment, suspension or exclusion from Medicare, Medicaid and other government reimbursement programs, any of which would have a material adverse effect on our business.
Changes in healthcare laws and regulations and new interpretations of existing laws and regulations may affect permissible activities, the relative costs associated with doing business, and reimbursement amounts paid by federal, state and other third-party payors. There have been and will continue to be regulatory initiatives affecting our business and we cannot predict the extent to which future legislation and regulatory changes could have a material adverse effect on our business.
We are subject to burdensome and complex billing and record-keeping requirements in order to substantiate our claims for payment under federal, state and commercial healthcare reimbursement programs, and our failure to comply with existing requirements, or changes in those requirements or interpretations thereof, could adversely affect our business, financial condition and operating results.
We are subject to burdensome and complex billing and record-keeping requirements in order to substantiate our claims for payment under federal, state and commercial healthcare reimbursement programs. Our records also are subject to routine and other reviews by third-party payors, which can result in delays in payments or refunds of paid claims. For example, we have also experienced a significant increase in pre-payment reviews of our claims by the Durable Medical Equipment Medicare Administrative Contractors, which has caused substantial delays in the collection of our Medicare accounts receivable as well as related amounts due under supplemental insurance plans.
Current law provides for a significant expansion of the government’s auditing and oversight of suppliers who care for patients covered by various government healthcare programs. Examples of this expansion include audit programs being implemented by the Durable Medical Equipment Medicare Administrative Contractors, the Zone Program Integrity Contractors, the Recovery Audit Contractors, and the Comprehensive Error Rate Testing contractors, operating under the direction of the Centers for Medicare & Medicaid Services.
We have been informed by these auditors that healthcare providers and suppliers of certain durable medical equipment product categories are expected to experience further increased scrutiny from these audit programs. When a government auditor ascribes a high billing error rate to one or more of our locations, it generally results in protracted pre-payment claims review, payment delays, refunds and other payments to the government and/or our need to request more documentation from providers than has historically been required. It may also result in additional audit activity in other company locations in that state or Durable Medical Equipment Medicare Administrative Contractors jurisdiction. We cannot currently predict the adverse impact that these audits, methodologies and interpretations might have on our business, financial condition or operating results, but such impact could be material.
-18-
We are subject to significant regulation by numerous government agencies, including the U.S. Food and Drug Administration, or FDA. We cannot market or commercially distribute our products without obtaining and maintaining necessary regulatory clearances or approvals.
Our Inogen concentrators are medical devices subject to extensive regulation in the United States and in the foreign markets where we distribute our products. The FDA and other U.S. and foreign governmental agencies regulate, among other things, with respect to medical devices:
|
• |
design, development and manufacturing; |
|
• |
testing, labeling, content and language of instructions for use and storage; |
|
• |
clinical trials; |
|
• |
product safety; |
|
• |
marketing, sales and distribution; |
|
• |
pre-market clearance and approval; |
|
• |
record keeping procedures; |
|
• |
advertising and promotion; |
|
• |
recalls and field safety corrective actions; |
|
• |
post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury; |
|
• |
post-market approval studies; and |
|
• |
product import and export. |
Before we can market or sell a medical device in the United States, we must obtain either clearance from the FDA under Section 510(k) of the Federal Food, Drug, and Cosmetic Act, or the FDCA, or approval of a pre-market approval application from the FDA, unless an exemption from pre-market review applies. In the 510(k) clearance process, the FDA must determine that a proposed device is “substantially equivalent” to a device legally on the market, known as a “predicate” device, with respect to intended use, technology and safety and effectiveness, in order to clear the proposed device for marketing. Clinical data is sometimes required to support substantial equivalence. The pre-market approval pathway requires an applicant to demonstrate the safety and effectiveness of the device based, in part, on extensive data, including, but not limited to, technical, preclinical, clinical trial, manufacturing and labeling data. The pre-market approval process is typically required for devices that are deemed to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices. Products that are approved through a pre-market approval application generally need FDA approval before they can be modified. Similarly, some modifications made to products cleared through a 510(k) may require a new 510(k). Both the 510(k) and pre-market approval processes can be expensive and lengthy and require the payment of significant fees, unless an exemption applies. The FDA’s 510(k) clearance process usually takes from three to twelve months, but may take longer. The process of obtaining a pre-market approval is much more costly and uncertain than the 510(k) clearance process and generally takes from one to three years, or longer, from the time the application is submitted to the FDA until an approval is obtained. The process of obtaining regulatory clearances or approvals to market a medical device can be costly and time consuming, and we may not be able to obtain these clearances or approvals on a timely basis, if at all.
In the United States, our currently commercialized products are marketed pursuant to pre-market clearance under Section 510(k) of the FDCA. If the FDA requires us to go through a lengthier, more rigorous examination for future products or modifications to existing products than we had expected, our product introductions or modifications could be delayed or canceled, which could cause our sales to decline. In addition, the FDA may determine that future products will require the more costly, lengthy and uncertain pre-market approval process. Although we do not currently market any devices under a pre-market approval, the FDA may demand that we obtain a pre-market approval prior to marketing certain of our future products. In addition, if the FDA disagrees with our determination that a product we currently market is subject to an exemption from pre-market review, the FDA may require us to submit a 510(k) or pre-market approval application in order to continue marketing the product. Further, even with respect to those future products where a pre-market approval is not required, we cannot assure you that we will be able to obtain the 510(k) clearances with respect to those products.
-19-
The FDA can delay, limit or deny clearance or approval of a device for many reasons, including:
|
• |
we may not be able to demonstrate to the FDA’s satisfaction that our products are safe and effective for their intended users; |
|
• |
the data from our pre-clinical studies and clinical trials may be insufficient to support clearance or approval, where required; and |
|
• |
the manufacturing process or facilities we use may not meet applicable requirements. |
In addition, the FDA may change its clearance and approval policies, adopt additional regulations or revise existing regulations, or take other actions which may prevent or delay approval or clearance of our products under development or impact our ability to modify our currently approved or cleared products on a timely basis. For example, in response to industry and healthcare provider concerns regarding the predictability, consistency and rigor of the 510(k) regulatory pathway, the FDA initiated an evaluation of the program, and in January 2011, announced several proposed actions intended to reform the review process governing the clearance of medical devices. The FDA intends these reform actions to improve the efficiency and transparency of the clearance process, as well as bolster patient safety. Some of these proposals, if enacted, could impose additional regulatory requirements upon us which could delay our ability to obtain new 510(k) clearances, increase the costs of compliance or restrict our ability to maintain our current clearances. In addition, as part of the Food and Drug Administration Safety and Innovation Act, Congress reauthorized the Medical Device User Fee Amendments with various FDA performance goal commitments and enacted several “Medical Device Regulatory Improvements” and miscellaneous reforms which are further intended to clarify and improve medical device regulation both pre- and post-market.
Medical devices may only be promoted and sold for the indications for which they are approved or cleared. In addition, even if the FDA has approved or cleared a product, it can take action affecting such product approvals or clearances if serious safety or other problems develop in the marketplace. Delays in obtaining clearances or approvals could adversely affect our ability to introduce new products or modifications to our existing products in a timely manner, which would delay or prevent commercial sales of our products. Additionally, the FDA and other regulatory authorities have broad enforcement powers. Regulatory enforcement or inquiries, or other increased scrutiny on us, could affect the perceived safety and efficacy of our products and dissuade our customers from using our products.
If we modify our FDA cleared devices, we may need to seek additional clearances or approvals, which, if not granted, would prevent us from selling our modified products.
Our Inogen One systems and Inogen At Home system have received pre-market clearance under Section 510(k) of the FDCA. The modifications made to our Inogen One G2 and Inogen One G3 systems represent non-significant modifications to the original Inogen One system, have the same indications for use, and are covered under our initial Inogen One 510(k) clearance. Any modifications to a 510(k)-cleared device that could significantly affect its safety or effectiveness, or would constitute a major change in its intended use, manufacture, design, components, or technology requires the submission and clearance of a new 510(k) pre-market notification or, possibly, pre-market approval. The FDA requires every manufacturer to make this determination in the first instance, but the FDA may review any manufacturer’s decision. The FDA may not agree with our decisions regarding whether new clearances or approvals are necessary. We have modified some of our 510(k) cleared products, and have determined based on our review of the applicable FDA guidance that in certain instances new 510(k) clearances or pre-market approval are not required. If the FDA disagrees with our determination and requires us to submit new 510(k) notifications or pre-market approval for modifications to our previously cleared products for which we have concluded that new clearances or approvals are unnecessary, we may be required to cease marketing or to recall the modified product until we obtain clearance or approval, and we may be subject to significant regulatory fines or penalties.
Furthermore, the FDA’s ongoing review of the 510(k) program may make it more difficult for us to make modifications to our previously cleared products, either by imposing more strict requirements on when a manufacturer must submit a new 510(k) for a modification to a previously cleared product, or by applying more onerous review criteria to such submissions. Specifically, pursuant to the Food and Drug Administration Safety and Innovation Act, which was signed into law in July 2012, the FDA was obligated to prepare a report for Congress on the FDA’s approach for determining when a new 510(k) will be required for modifications or changes to a previously cleared device. The FDA issued this report in 2014 and indicated that manufacturers should continue to adhere to the FDA’s 1997 Guidance on this topic when making a determination as to whether or not a new 510(k) is required for a change or modification to a device. However, the practical impact of the FDA’s continuing scrutiny of these issues remains unclear.
-20-
If we fail to comply with FDA or state regulatory requirements, we can be subject to enforcement action.
The regulations to which we are subject are complex and have become more stringent over time. Regulatory changes could result in restrictions on our ability to continue or expand our operations, higher than anticipated costs or lower than anticipated sales. Even after we have obtained the proper regulatory clearance or approval to market a product, we have ongoing responsibilities under FDA regulations. The FDA and state authorities have broad enforcement powers. Our failure to comply with applicable regulatory requirements could result in enforcement action by the FDA or state agencies, which may include any of the following sanctions:
|
• |
warning letters, fines, injunctions, consent decrees and civil penalties; |
|
• |
recalls, termination of distribution, or seizure of our products; |
|
• |
operating restrictions or partial suspension or total shutdown of production; |
|
• |
delays in the introduction of products into the market; |
|
• |
refusal to grant our requests for future 510(k) clearances or approvals of new products, new intended uses, or modifications to exiting products; |
|
• |
withdrawals or suspensions of current 510(k) clearances or approvals, resulting in prohibitions on sales of our products; and |
|
• |
criminal prosecution. |
Any of these sanctions could result in higher than anticipated costs or lower than anticipated sales and have a material adverse effect on our reputation, business, results of operations and financial condition.
A recall of our products, either voluntarily or at the direction of the FDA or another governmental authority, or the discovery of serious safety issues with our products that leads to corrective actions, could have a significant adverse impact on us.
Medical devices, such as our Inogen concentrators, can experience performance problems in the field that require review and possible corrective action by us or the product manufacturer. We cannot provide assurance that component failures, manufacturing errors, design defects and/or labeling inadequacies, which could result in an unsafe condition or injury to the operator or the patient will not occur. The FDA and similar foreign governmental authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture of a product or in the event that a product poses an unacceptable risk to health. Manufacturers may also, under their own initiative, recall a product if any material deficiency in a device is found or withdraw a product to improve device performance or for other reasons. A government-mandated or voluntary recall by us or one of our distributors could occur as a result of an unacceptable risk to health, component failures, manufacturing errors, design or labeling defects or other deficiencies and issues. Similar regulatory agencies in other countries have similar authority to recall devices because of material deficiencies or defects in design or manufacture that could endanger health. Any recall would divert management attention and financial resources, could cause the price of our stock to decline and expose us to product liability or other claims and harm our reputation with customers. A recall involving our Inogen concentrators could be particularly harmful to our business, financial and operating results.
In addition, under the FDA’s medical device reporting regulations, we are required to report to the FDA any incident in which our product may have caused or contributed to a death or serious injury or in which our product malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury. Repeated product malfunctions may result in a voluntary or involuntary product recall. Depending on the corrective action we take to redress a product’s deficiencies or defects, the FDA may require, or we may decide, that we will need to obtain new approvals or clearances for the device before we may market or distribute the corrected device. Seeking such approvals or clearances may delay our ability to replace the recalled devices in a timely manner. Moreover, if we do not adequately address problems associated with our devices, we may face additional regulatory enforcement action, including FDA warning letters, product seizure, injunctions, administrative penalties, or civil or criminal fines. We may also be required to bear other costs or take other actions that may have a negative impact on our sales as well as face significant adverse publicity or regulatory consequences, which could harm our business, including our ability to market our products in the future.
Any adverse event involving our products, whether in the United States or abroad, could result in future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection, mandatory recall or other enforcement action. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time and capital, distract management from operating our business and may harm our reputation and financial results.
If we or our component manufacturers fail to comply with the FDA’s Quality System Regulation, our manufacturing operations could be interrupted, and our product sales and operating results could suffer.
We and our component manufacturers are required to comply with the FDA’s Quality System Regulation, or QSR, which covers the procedures and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage
-21-
and shipping of our devices. The FDA audits compliance with the QSR through periodic announced and unannounced inspections of manufacturing and other facilities. We and our component manufacturers have been, and anticipate in the future being, subject to such inspections. Although we believe our manufacturing facilities and those of our component manufacturers are in compliance with the QSR, we cannot provide assurance that any future inspection will not result in adverse findings. If our manufacturing facilities or those of any of our component manufacturers or suppliers are found to be in violation of applicable laws and regulations, or we or our manufacturers or suppliers fail to take satisfactory corrective action in response to an adverse inspection, the FDA could take enforcement action, including any of the following sanctions:
|
• |
untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
|
• |
customer notifications or repair, replacement, refunds, recall, detention or seizure of our products; |
|
• |
operating restrictions or partial suspension or total shutdown of production; |
|
• |
refusing or delaying our requests for 510(k) clearance or pre-market approval of new products or modified products; |
|
• |
withdrawing 510(k) clearances or pre-market approvals that have already been granted; |
|
• |
refusal to grant export approval for our products; or |
|
• |
criminal prosecution. |
Any of these sanctions could adversely affect our business, financial conditions and operating results.
Outside the United States, our products and operations are also often required to comply with standards set by industrial standards bodies, such as the International Organization for Standardization, or ISO. Foreign regulatory bodies may evaluate our products or the testing that our products undergo against these standards. The specific standards, types of evaluation and scope of review differ among foreign regulatory bodies. If we fail to adequately comply with any of these standards, a foreign regulatory body may take adverse actions similar to those within the power of the FDA. Any such action may harm our reputation and could have an adverse effect on our business, results of operations and financial condition.
If we fail to obtain and maintain regulatory approval in foreign jurisdictions, our market opportunities will be limited.
Approximately 19.6% and 23.2% of our revenue was from sales outside of the United States for the six months ended June 30, 2014 and June 30, 2013, respectively. As of June 30, 2014, we sold our products in 44 countries outside of the United States through distributors or directly to large “house” accounts. In order to market our products in the European Union or other foreign jurisdictions, we must obtain and maintain separate regulatory approvals and comply with numerous and varying regulatory requirements. The approval procedure varies from country to country and can involve additional testing. The time required to obtain approval abroad may be longer than the time required to obtain FDA clearance. The foreign regulatory approval process includes many of the risks associated with obtaining FDA clearance and we may not obtain foreign regulatory approvals on a timely basis, if at all. FDA clearance does not ensure approval by regulatory authorities in other countries, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries. However, the failure to obtain clearance or approval in one jurisdiction may have a negative impact on our ability to obtain clearance or approval elsewhere. If we do not obtain or maintain necessary approvals to commercialize our products in markets outside the United States, it would negatively affect our overall market penetration.
We may be subject to fines, penalties or injunctions if we are determined to be promoting the use of our products for unapproved or “off-label” uses, resulting in damage to our reputation and business.
Our promotional materials and training methods must comply with the FDA and other applicable laws and regulations, including the prohibition of the promotion of a medical device for a use that has not been cleared or approved by the FDA. Use of a device outside its cleared or approved indications is known as “off-label” use. Physicians may use our products off-label, as the FDA does not restrict or regulate a physician’s choice of treatment within the practice of medicine. If the FDA determines that our promotional materials or training constitutes promotion of an off-label use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, which could have an adverse impact on our reputation and financial results.
-22-
Failure to comply with the Federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, the Health Information Technology for Economic and Clinical Health Act, or HITECH Act, and implementing regulations (including the final omnibus rule published on January 25, 2013) affecting the transmission, security and privacy of health information could result in significant penalties.
Numerous federal and state laws and regulations, including HIPAA and the HITECH Act, govern the collection, dissemination, security, use and confidentiality of patient-identifiable health information. HIPAA and the HITECH Act require us to comply with standards for the use and disclosure of health information within our company and with third parties. The Privacy Standards and Security Standards under HIPAA establish a set of basic national privacy and security standards for the protection of individually identifiable health information by health plans, healthcare clearinghouses and certain healthcare providers, referred to as covered entities, and the business associates with whom such covered entities contract for services. Notably, whereas HIPAA previously directly regulated only these covered entities, the HITECH Act, which was signed into law as part of the stimulus package in February 2009, makes certain of HIPAA’s privacy and security standards also directly applicable to covered entities’ business associates. As a result, both covered entities and business associates are now subject to significant civil and criminal penalties for failure to comply with Privacy Standards and Security Standards.
HIPAA and the HITECH Act also include standards for common healthcare electronic transactions and code sets, such as claims information, plan eligibility, payment information and the use of electronic signatures, and privacy and electronic security of individually identifiable health information. Covered entities, such as healthcare providers, are required to conform to such transaction set standards pursuant to HIPAA.
HIPAA requires healthcare providers like us to develop and maintain policies and procedures with respect to protected health information that is used or disclosed, including the adoption of administrative, physical and technical safeguards to protect such information. The HITECH Act expands the notification requirement for breaches of patient-identifiable health information, restricts certain disclosures and sales of patient-identifiable health information and provides a tiered system for civil monetary penalties for HIPAA violations. The HITECH Act also increased the civil and criminal penalties that may be imposed against covered entities, business associates and possibly other persons and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney fees and costs associated with pursuing federal civil actions. Additionally, certain states have adopted comparable privacy and security laws and regulations, some of which may be more stringent than HIPAA.
If we do not comply with existing or new laws and regulations related to patient health information, we could be subject to criminal or civil sanctions. New health information standards, whether implemented pursuant to HIPAA, the HITECH Act, congressional action or otherwise, could have a significant effect on the manner in which we handle healthcare related data and communicate with payors, and the cost of complying with these standards could be significant.
The 2013 final HITECH omnibus rule modifies the breach reporting standard in a manner that will likely make more data security incidents qualify as reportable breaches. Any liability from a failure to comply with the requirements of HIPAA or the HITECH Act could adversely affect our financial condition. The costs of complying with privacy and security related legal and regulatory requirements are burdensome and could have a material adverse effect on our results of operations. These new provisions, as modified, will be subject to interpretation by various courts and other governmental authorities, thus creating potentially complex compliance issues for us, as well as our clients and strategic partners. In addition, we are unable to predict what changes to the HIPAA Privacy Standards and Security Standards might be made in the future or how those changes could affect our business. Any new legislation or regulation in the area of privacy and security of personal information, including personal health information, could also adversely affect our business operations.
Regulations requiring the use of “standard transactions” for healthcare services issued under HIPAA may negatively impact our profitability and cash flows.
Pursuant to HIPAA, final regulations have been implemented to improve the efficiency and effectiveness of the healthcare system by facilitating the electronic exchange of information in certain financial and administrative transactions while protecting the privacy and security of the information exchanged.
The HIPAA transaction standards are complex, and subject to differences in interpretation by third-party payors. For instance, some third-party payors may interpret the standards to require us to provide certain types of information, including demographic information not usually provided to us by physicians. As a result of inconsistent application of transaction standards by third-party payors or our inability to obtain certain billing information not usually provided to us by physicians, we could face increased costs and complexity, a temporary disruption in accounts receivable and ongoing reductions in reimbursements and net revenue. In addition, requirements for additional standard transactions, such as claims attachments or use of a national provider identifier, could prove technically difficult, time-consuming or expensive to implement, all of which could harm our business.
-23-
If we fail to comply with state and federal fraud and above laws, including anti-kickback, false claims and anti-inducement laws, we could face substantial penalties and our business, operations, and financial condition could be adversely affected.
The federal anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce or in return for purchasing, leasing, ordering, or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid, or other federal financed healthcare programs. Although there are a number of statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution, the exceptions and safe harbors are drawn narrowly, and any remuneration to or from a prescriber or purchaser of healthcare products or services may be subject to scrutiny if they do not qualify for an exception or safe harbor. Our practices may not in all cases meet all of the criteria for safe harbor protection from anti-kickback liability.
Federal false claims laws prohibit any person from knowingly presenting or causing to be presented a false claim for payment to the federal government, or knowingly making or causing to be made a false statement to get a false claim paid. The majority of states also have statutes or regulations similar to the federal anti-kickback law and false claims laws, which apply to items or services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of payor. These false claims statutes allow any person to bring suit in the name of the government alleging false and fraudulent claims presented to or paid by the government (or other violations of the statutes) and to share in any amounts paid by the entity to the government in fines or settlement. Such suits, known as qui tam actions, have increased significantly in the healthcare industry in recent years. Sanctions under these federal and state laws may include civil monetary penalties, exclusion of a manufacturer’s products from reimbursement under government programs, criminal fines and imprisonment. In addition, the recently enacted Patient Protection and Affordable Care Act, among other things, amends the intent requirement of the federal anti-kickback and criminal healthcare fraud statutes. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the Patient Protection and Affordable Care Act provides that the government may assert that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the false claims statutes. Because of the breadth of these laws and the narrowness of the safe harbors and exceptions, it is possible that some of our business activities could be subject to challenge under one or more of such laws. Such a challenge, regardless of the outcome, could have a material adverse effect on our business, business relationships, reputation, financial condition and results of operations.
The Patient Protection and Affordable Care Act also imposes new reporting and disclosure requirements on device and drug manufacturers for any “transfer of value” made or distributed to prescribers and other healthcare providers. Device and drug manufacturers will also be required to report and disclose any investment interests held by physicians and their immediate family members during the preceding calendar year. Failure to submit required information may result in civil monetary penalties of up to an aggregate of $150,000 per year (and up to an aggregate of $1 million per year for “knowing failures”), for all payments, transfers of value or ownership or investment interests not reported in an annual submission. As of August 1, 2013, manufacturers are required to collect data, and were required to submit their first data reports to the Centers for Medicare & Medicaid Services by March 31, 2014 and by the 90th day of each calendar year thereafter.
In addition, there has been a recent trend of increased federal and state regulation of payments made to physicians. Certain states, mandate implementation of compliance programs and/or the tracking and reporting of gifts, compensation and other remuneration to physicians. The shifting compliance environment and the need to build and maintain robust and expandable systems to comply with different compliance and/or reporting requirements in multiple jurisdictions increase the possibility that a healthcare company many violate one or more of the requirements.
The Federal Civil Monetary Penalties Law prohibits the offering or giving of remuneration to a Medicare or Medicaid beneficiary that the person knows or should know is likely to influence the beneficiary’s selection of a particular supplier of items or services reimbursable by a Federal or state governmental healthcare program. We sometimes offer customers various discounts and other financial incentives in connection with the sales of our products. While it is our intent to comply with all applicable laws, the government may find that our marketing activities violate the Civil Monetary Penalties Law. If we are found to be in noncompliance, we could be subject to civil money penalties of up to $10,000 for each wrongful act, assessment of three times the amount claimed for each item or service and exclusion from the federal or state healthcare programs.
The scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform, especially in light of the lack of applicable precedent and regulations. If our operations are found to be in violation of any of the laws described above or any other government regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines and the curtailment or restricting of our operations. Any penalties, damages, fines, curtailment or restructuring or our operations could harm our ability to operate our business and our financial results. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from operation of our business. Moreover, achieving and sustaining compliance with applicable federal and state fraud laws may prove costly.
-24-
Foreign governments tend to impose strict price controls, which may adversely affect our future profitability.
As of June 30, 2014 we sold our products in 44 countries outside the United States through distributors or directly to large “house” accounts. In some foreign countries, particularly in the European Union, the pricing of medical devices is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we may be required to supply data that compares the cost-effectiveness of our Inogen One systems to other available oxygen therapies. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, it may not be profitable to sell our products in certain foreign countries, which would negatively affect the long-term growth of our business.
Our business activities involve the use of hazardous materials, which require compliance with environmental and occupational safety laws regulating the use of such materials. If we violate these laws, we could be subject to significant fines, liabilities or other adverse consequences.
Our research and development programs as well as our manufacturing operations involve the controlled use of hazardous materials. Accordingly, we are subject to federal, state and local laws governing the use, handling and disposal of these materials. Although we believe that our safety procedures for handling and disposing of these materials comply in all material respects with the standards prescribed by state and federal regulations, we cannot completely eliminate the risk of accidental contamination or injury from these materials. In the event of an accident or failure to comply with environmental laws, we could be held liable for resulting damages, and any such liability could exceed our insurance coverage.
Risks related to our intellectual property
If we are unable to secure and maintain patent or other intellectual property protection for the intellectual property used in our products, we will lose a significant competitive advantage, which may adversely affect our future profitability.
Our commercial success depends, in part, on obtaining and maintaining patent and other intellectual property protection for the technologies used in our products. The patent positions of medical device companies, including ours, can be highly uncertain and involve complex and evolving legal and factual questions. Furthermore, we might in the future opt to license intellectual property from other parties. If we, or the other parties from whom we would license intellectual property, fail to obtain and maintain adequate patent or other intellectual property protection for intellectual property used in our products, or if any protection is reduced or eliminated, others could use the intellectual property used in our products, resulting in harm to our competitive business position. In addition, patent and other intellectual property protection may not:
|
• |
prevent our competitors from duplicating our products; |
|
• |
prevent our competitors from gaining access to our proprietary information and technology; or |
|
• |
permit us to gain or maintain a competitive advantage. |
Any of our patents may be challenged, invalidated, circumvented or rendered unenforceable. We cannot provide assurance that we will be successful should one or more of our patents be challenged for any reason. If our patent claims are rendered invalid or unenforceable, or narrowed in scope, the patent coverage afforded our products could be impaired, which could make our products less competitive.
As of September 30, 2014, we had five pending U.S. patent applications, 27 issued U.S. patents and one issued Canadian patent relating to the design and construction of our oxygen concentrators and our intelligent delivery technology. We cannot specify which of these patents individually or as a group will permit us to gain or maintain a competitive advantage. U.S. patents and patent applications may be subject to interference proceedings, and U.S. patents may be subject to re-examination, inter partes review, post-grant review, and derivation proceedings in the U.S. Patent and Trademark Office. Foreign patents may be subject to opposition or comparable proceedings in the corresponding foreign patent offices. Any of these proceedings could result in loss of the patent or denial of the patent application, or loss or reduction in the scope of one or more of the claims of the patent or patent application. Changes in either patent laws or in interpretations of patent laws may also diminish the value of our intellectual property or narrow the scope of our protection. Interference, re-examination, inter partes review, and opposition proceedings may be costly and time consuming, and we, or the other parties from whom we might potentially license intellectual property, may be unsuccessful in defending against such proceedings. Thus, any patents that we own or might license may provide limited or no protection against competitors. In addition, our pending patent applications and those we may file in the future may have claims narrowed during prosecution or may not result in patents being issued. Even if any of our pending or future applications are issued, they may not provide us with adequate protection or any competitive advantages. Our patents and patent applications are directed to particular aspects of our products. Other parties may develop and obtain patent protection for more effective technologies, designs or methods for oxygen therapy. If these developments were to occur, it would likely have an adverse effect on our sales. Our ability to develop additional patentable technology is also uncertain.
-25-
Non-payment or delay in payment of patent fees or annuities, whether intentional or unintentional, may also result in the loss of patents or patent rights important to our business. Many countries, including certain countries in Europe, have compulsory licensing laws under which a patent owner may be compelled to grant licenses to other parties. In addition, many countries limit the enforceability of patents against other parties, including government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of the patent. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as do the laws of the United States, particularly in the field of medical products and procedures.
Our products could infringe the intellectual property rights of others, which may lead to patent and other intellectual property litigation that could itself be costly, could result in the payment of substantial damages or royalties, prevent us from using technology that is essential to our products, and/or force us to discontinue selling our products.
The medical device industry in general has been characterized by extensive litigation and administrative proceedings regarding patent infringement and intellectual property rights. Our competitors hold a significant number of patents relating to oxygen therapy devices and products. From time to time, we have commenced litigation to enforce our intellectual property rights. For example, we have pursued litigation against Inova Labs Inc. for infringement of two of our patents seeking damages, injunctive relief, costs, and attorneys’ fees. An adverse decision in this action or in any other legal action could limit our ability to assert our intellectual property rights, limit the value of our technology or otherwise negatively impact our business, financial condition and results of operations.
Monitoring unauthorized use of our intellectual property is difficult and costly. Unauthorized use of our intellectual property may have occurred or may occur in the future. Although we have taken steps to minimize the risk of this occurring, any such failure to identify unauthorized use and otherwise adequately protect our intellectual property would adversely affect our business. Moreover, if we are required to commence litigation, whether as a plaintiff or defendant as has occurred with Inova Labs Inc., not only will this be time-consuming, but we will also be forced to incur significant costs and divert our attention and efforts of our employees, which could, in turn, result in lower revenue and higher expenses.
We cannot provide assurance that our products or methods do not infringe the patents or other intellectual property rights of third parties and if our business is successful, the possibility may increase that others will assert infringement claims against us.
Determining whether a product infringes a patent involves complex legal and factual issues, and the outcome of a patent litigation action is often uncertain. We have not conducted an extensive search of patents issued or assigned to other parties, including our competitors, and no assurance can be given that patents containing claims covering our products, parts of our products, technology or methods do not exist, have not been filed or could not be filed or issued. Because of the number of patents issued and patent applications filed in our technical areas, our competitors or other parties may assert that our products and the methods we employ in the use of our products are covered by U.S. or foreign patents held by them. In addition, because patent applications can take many years to issue and because publication schedules for pending applications vary by jurisdiction and some companies opt not to publish their patent applications, there may be applications now pending of which we are unaware and which may result in issued patents that our current or future products infringe. Also, because the claims of published patent applications can change between publication and patent grant, there may be published patent applications that may ultimately issue with claims that we infringe. There could also be existing patents that one or more of our products or parts may infringe and of which we are unaware. As the number of competitors in the market for oxygen products and as the number of patents issued in this area grows, the possibility of patent infringement claims against us increases. In certain situations, we may determine that it is in our best interests to voluntarily challenge a party’s products or patents in litigation or other proceedings, including patent re-examinations, or inter partes reviews. As a result, we may become involved in unwanted litigation that could be costly, result in diversion of management’s attention, require us to pay damages and force us to discontinue selling our products.
Infringement and other intellectual property claims and proceedings brought against us, whether successful or not, could result in substantial costs and harm to our reputation. Such claims and proceedings can also distract and divert management and key personnel from other tasks important to the success of the business. We cannot be certain that we will successfully defend against allegations of infringement of patents and intellectual property rights of others. In the event that we become subject to a patent infringement or other intellectual property lawsuit and if the other party’s patents or other intellectual property were upheld as valid and enforceable and we were found to infringe the other party’s patents or violate the terms of a license to which we are a party, we could be required to do one or more of the following:
|
• |
cease selling or using any of our products that incorporate the asserted intellectual property, which would adversely affect our revenue; |
|
• |
pay damages for past use of the asserted intellectual property, which may be substantial; |
-26-
|
• |
obtain a license from the holder of the asserted intellectual property, which license may not be available on reasonable terms, if at all, and which could reduce profitability; and |
|
• |
redesign or rename, in the case of trademark claims, our products to avoid infringing the intellectual property rights of third parties, which may not be possible and could be costly and time-consuming if it is possible to do so. |
If we are unable to prevent unauthorized use or disclosure of trade secrets, unpatented know-how and other proprietary information, our ability to compete will be harmed.
We rely on a combination of trade secrets, copyrights, trademarks, confidentiality agreements and other contractual provisions and technical security measures to protect certain aspects of our technology, especially where we do not believe that patent protection is appropriate or obtainable. We require our employees and consultants to execute confidentiality agreements in connection with their employment or consulting relationships with us. We also require our employees and consultants to disclose and assign to us all inventions conceived during the term of their employment or engagement while using our property or that relate to our business. We also require our corporate partners, outside scientific collaborators and sponsored researchers, advisors and others with access to our confidential information to sign confidentiality agreements. We also have taken precautions to initiate reasonable safeguards to protect our information technology systems. However, these measures may not be adequate to safeguard our proprietary intellectual property and conflicts may, nonetheless, arise regarding ownership of inventions. Such conflicts may lead to the loss or impairment of our intellectual property or to expensive litigation to defend our rights against competitors who may be better funded and have superior resources. Our employees, consultants, contractors, outside clinical collaborators and other advisors may unintentionally or willfully disclose our confidential information to competitors. In addition, confidentiality agreements may be unenforceable or may not provide an adequate remedy in the event of unauthorized disclosure. Enforcing a claim that a third party illegally obtained and is using our trade secrets is expensive and time-consuming, and the outcome is unpredictable. Moreover, our competitors may independently develop equivalent knowledge, methods and know-how. Unauthorized parties may also attempt to copy or reverse engineer certain aspects of our products that we consider proprietary, and in such cases we could not assert any trade secret rights against such party. As a result, other parties may be able to use our proprietary technology or information, and our ability to compete in the market would be harmed.
We have registered the trademarks Inogen; Inogen One; Inogen One G2; Oxygenation; Live Life in Moments, not Minutes; Never Run Out of Oxygen; Oxygen Therapy on Your Terms; Oxygen. Anytime, Anywhere; Reclaim Your Independence; Intelligent Delivery Technology; and the Inogen design with the United States Patent and Trademark Office. We have applied with the United States Patent and Trademark Office to register the trademark Inogen at Home. We have registered the trademark Inogen in Australia, Canada, China, Mexico, and in Europe (European Community registration). We have registered the trademark Inogen One in Australia, Canada, China, Mexico, and in Europe (European Community registration). We have registered the trademark Satellite Conserver in Canada and China. We have registered the trademark Inogen at Home in Europe (European Community registration).
We may be subject to damages resulting from claims that our employees or we have wrongfully used or disclosed alleged trade secrets of other companies.
Many of our employees were previously employed at other medical device companies focused on the development of oxygen therapy products, including our competitors. Although no claims against us are currently pending, we may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights. Even if we are successful in defending against these claims, litigation could result in substantial costs, damage to our reputation and be a distraction to management.
Risks related to being a public company
We will incur increased costs as a result of operating as a public company and our management will be required to devote substantial time to new compliance initiatives and corporate governance practices.
On February 20, 2014 we completed our initial public offering. As a public company, and increasingly after we are no longer an “emerging growth company,” we will incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, the Sarbanes-Oxley Act and rules subsequently implemented by the SEC and the NASDAQ Global Select Market impose numerous requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Also, the Securities Exchange Act of 1934, as amended, or the Exchange Act, requires, among other things, that we file annual, quarterly and current reports with respect to our business and operating results. Our management and other personnel will need to devote a substantial amount of time to compliance with these laws and regulations. These requirements have increased and will continue to increase our legal, accounting, and financial compliance costs and have made and will continue to make some activities more time consuming and costly. For example, we expect these rules and regulations to
-27-
make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to incur substantial costs to maintain the same or similar coverage. These rules and regulations could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors or our board committees or as executive officers.
Overall, we estimate that our incremental costs resulting from operating as a public company, including compliance with these rules and regulations, may be between $1.5 million and $3.0 million per year. However, these rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
The Sarbanes-Oxley Act requires, among other things, that we assess the effectiveness of our internal control over financial reporting annually and the effectiveness of our disclosure controls and procedures quarterly. In particular, Section 404(a) of the Sarbanes-Oxley Act, or Section 404(a), will require us to perform system and process evaluation and testing of our internal control over financial reporting to allow management to report on the effectiveness of our internal control over financial reporting. Section 404(b) of Sarbanes-Oxley Act, or Section 404(b), also requires our independent registered public accounting firm to attest to the effectiveness of our internal control over financial reporting. As an “emerging growth company” we expect to avail ourselves of the exemption from the requirement that our independent registered public accounting firm attest to the effectiveness of our internal control over financial reporting under Section 404(b). However, we may no longer avail ourselves of this exemption when we are no longer an “emerging growth company.” When our independent registered public accounting firm is required to undertake an assessment of our internal control over financial reporting, the cost of our compliance with Section 404(b) will correspondingly increase. Our compliance with applicable provisions of Section 404 will require that we incur substantial accounting expense and expend significant management time on compliance-related issues as we implement additional corporate governance practices and comply with reporting requirements.
Furthermore, investor perceptions of our company may suffer if deficiencies are found, and this could cause a decline in the market price of our stock. Irrespective of compliance with Section 404, any failure of our internal control over financial reporting could have a material adverse effect on our stated operating results and harm our reputation. If we are unable to implement these requirements effectively or efficiently, it could harm our operations, financial reporting, or financial results and could result in an adverse opinion on our internal controls from our independent registered public accounting firm.
If we fail to maintain effective internal control over financial reporting in the future, the accuracy and timing of our financial reporting may be adversely affected.
No material weaknesses in internal control over financial reporting were identified in connection with the audit of our financial statements for the year ended December 31, 2013. However, our management and independent registered public accounting firm did not perform an evaluation of our internal control over financial reporting during any period in accordance with the provisions of the Sarbanes-Oxley Act. Had we and our independent registered public accounting firm performed an evaluation of our internal control over financial reporting in accordance with the provisions of the Sarbanes-Oxley Act, control deficiencies amounting to significant deficiencies or material weaknesses may have been identified. We cannot be certain as to when we will be able to implement the requirements of Section 404 of the Sarbanes-Oxley Act. If we fail to implement the requirements of Section 404 in a timely manner, we might be subject to sanctions or investigation by regulatory agencies such as the SEC. In addition, failure to comply with Section 404 or the report by us of a significant deficiency or material weakness may cause investors to lose confidence in our financial statements, and the trading price of our common stock may decline. If we fail to remedy any significant deficiency or material weakness, our financial statements may be inaccurate, our access to the capital markets may be restricted and the trading price of our ordinary shares may suffer.
We are an “emerging growth company” and the reduced disclosure requirements applicable to emerging growth companies may make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the 2012 Jumpstart Our Business Startups (JOBS) Act, and we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced financial disclosure obligations, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and any golden parachute payments not previously approved. We may take advantage of these provisions for up to five years or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company upon the earliest to occur of: the last day of the fiscal year in which we have more than $1.0 billion in annual revenue; the date we qualify as a large accelerated filer, with at least $700 million of equity securities held by non-affiliates; the issuance, in any three-year period, by us of more than $1.0 billion in non-convertible debt securities; and the last day of the fiscal year ending after the fifth anniversary of our initial public offering. We may choose to take advantage of some but not all of these reduced reporting burdens. If we take advantage of any of these reduced reporting burdens in future filings, the information that we
-28-
provide our security holders may be different than you might get from other public companies in which you hold equity interests. In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards, delaying the adoption of these accounting standards until they would apply to private companies. We have elected to avail ourselves of this exemption and, as a result, our financial statements may not be comparable to the financial statements of issuers who are required to comply with the effective dates for new or revised accounting standards that are applicable to public companies. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be reduced or more volatile.
Risks related to our common stock and this offering
Our stock price will fluctuate significantly, and you may not be able to resell your shares at or above the offering price.
The trading price of our common stock has fluctuated and may continue to fluctuate substantially. Since shares of our common stock were sold in our initial public offering in February 2014 at a price of $16.00 per share, the reported high and low sales prices of our common stock ranged from $13.1201 to $24.50 through October 10, 2014. The trading price of our common stock depends on a number of factors, including those described in this “Risk factors” section, many of which are beyond our control and may not be related to our operating performance. These fluctuations could cause you to lose all or part of your investment in our common stock since you might be unable to sell your shares at or above the price you paid in this offering. Factors that could cause fluctuations in the trading price of our common stock include the following:
|
• |
actual or anticipated quarterly variation in our results of operations or the results of our competitors; |
|
• |
announcements by us or our competitors of new commercial products, significant contracts, commercial relationships or capital commitments; |
|
• |
issuance of new or changed securities analysts’ reports or recommendations for our stock; |
|
• |
developments or disputes concerning our intellectual property or other proprietary rights; |
|
• |
commencement of, or our involvement in, litigation; |
|
• |
market conditions in the oxygen therapy market; |
|
• |
reimbursement or legislative changes in the oxygen therapy market; |
|
• |
failure to complete significant sales; |
|
• |
manufacturing disruptions that could occur if we were unable to successfully expand our production in our current or an alternative facility; |
|
• |
any future sales of our common stock or other securities; |
|
• |
any major change to the composition of our board of directors or management; and |
|
• |
general economic conditions and slow or negative growth of our markets. |
The stock market in general and market prices for the securities of technology-based companies like ours in particular, have from time to time experienced volatility that often has been unrelated to the operating performance of the underlying companies. A certain degree of stock price volatility can be attributed to being a newly public company. These broad market and industry fluctuations may adversely affect the market price of our common stock, regardless of our operating performance. In several recent situations where the market price of a stock has been volatile, holders of that stock have instituted securities class action litigation against the company that issued the stock. If any of our stockholders were to bring a lawsuit against us, the defense and disposition of the lawsuit could be costly and divert the time and attention of our management and harm our operating results.
If securities or industry analysts do not publish research or publish unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will rely in part on the research and reports that equity research analysts publish about us and our business. We will not have any control of the analysts or the content and opinions included in their reports. The price of our stock could decline if one or more equity research analysts downgrade our stock or issue other unfavorable commentary or research. If one or more equity research analysts ceases coverage of our company or fails to publish reports on us regularly, demand for our stock could decrease, which in turn could cause our stock price or trading volume to decline.
-29-
Future sales of shares of our common stock, or the perception that such sales may occur, could cause our stock price to decline.
If our existing stockholders sell substantial amounts of their common stock in the public market, or are perceived by the public market as intending to sell, the trading price of our common stock could decline. As of September 30, 2014, we had outstanding a total of 18,436,802 shares of common stock, of which approximately 6.8 million shares are freely tradable without restriction in the public market and, which amount excludes 2,043,548 shares held by directors and executive officers and are subject to volume limitations under Rule 144 and the Securities Act of 1933, as amended, and various vesting agreements. Certain of our existing stockholders have demand and piggyback rights to require us to register with the SEC up to approximately shares of our common stock, excluding the shares of our common stock being offered in this prospectus. See “Description of capital stock – registration rights” for more information. If we register any of these shares of common stock, those stockholders would be able to sell those shares freely in the public market. In addition, after our initial public offering, we filed a registration statement under the Securities Act to register shares of our common stock that we may issue under our equity plans and, as of September 30, 2014, 2,678,328 shares of our common stock are issuable upon exercise of outstanding options that will become eligible for sale in the public market to the extent permitted by the vesting provisions thereunder.
In addition, in the future we may issue additional shares of our common stock or other equity or debt securities convertible into common stock in connection with a financing, acquisition or otherwise.
If any of these additional shares described are sold, or if it is perceived that they will be sold, in the public market, the trading price of our common stock could decline.
Our directors, executive officers and principal stockholders will continue to have substantial control over us after this offering and could limit your ability to influence the outcome of key transactions, including changes of control.
Following the completion of this offering, our executive officers, directors and stockholders who owned more than 5% of our outstanding common stock before this offering and their respective affiliates will beneficially own or control approximately % of the outstanding shares of our common stock, assuming no exercise of the underwriters’ option to purchase additional shares. Accordingly, these executive officers, directors and stockholders who owned more than 5% of our outstanding common stock before this offering and their respective affiliates, acting as a group, will have substantial influence over the outcome of corporate actions requiring stockholder approval, including the election of directors, any merger, consolidation or sale of all or substantially all of our assets or any other significant corporate transactions. These stockholders may also delay or prevent a change of control of us, even if such a change of control would benefit our other stockholders. The significant concentration of stock ownership may adversely affect the trading price of our common stock due to investors’ perception that conflicts of interest may exist or arise.
Anti-takeover provisions in our charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management and limit the market price of our common stock.
Provisions in our certificate of incorporation and bylaws may have the effect of delaying or preventing a change of control or changes in our management. Our amended and restated certificate of incorporation and amended and restated bylaws include provisions that:
|
• |
authorize our board of directors to issue, without further action by the stockholders, up to 10,000,000 shares of undesignated preferred stock; |
|
• |
require that any action to be taken by our stockholders be effected at a duly called annual or special meeting and not by written consent; |
|
• |
specify that special meetings of our stockholders can be called only by our board of directors, the Chairman of the board of directors, or the Chief Executive Officer; |
|
• |
establish an advance notice procedure for stockholder approvals to be brought before an annual meeting of our stockholders, including proposed nominations of persons for election to our board of directors; |
|
• |
establish that our board of directors is divided into three classes, Class I, Class II and Class III, with each class serving staggered three year terms; |
|
• |
provide that our directors may be removed only for cause; |
|
• |
provide that vacancies on our board of directors may be filled only by a majority of directors then in office, even though less than a quorum; |
|
• |
specify that no stockholder is permitted to cumulate votes at any election of directors; and |
|
• |
require a super-majority of votes to amend certain of the above-mentioned provisions. |
-30-
These provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, which is responsible for appointing the members of our management. In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which limits the ability of stockholders owning in excess of 15% of our outstanding voting stock to merge or combine with us.
We will not receive any proceeds from this offering, however, we continue to retain broad discretion in the use of the net proceeds from our initial public offering and may not use them effectively.
We will not receive any proceeds from this offering, however, we will continue to retain broad discretion in the application of the net proceeds from our initial public offering and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our common stock. We might not be able to yield a significant return, if any, on any investment of these net proceeds from the initial public offering. Stockholders will not have the opportunity to influence our management’s decisions on how to use the net proceeds, and our failure to apply these funds effectively could have a material adverse effect on our business and cause the price of our common stock to decline.
We have never paid dividends on our capital stock, and we do not anticipate paying any cash dividends in the foreseeable future.
We have paid no cash dividends on any of our classes of capital stock to date, have contractual restrictions against paying cash dividends and currently intend to retain our future earnings to fund the development and growth of our business. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
-31-
Special note regarding forward-looking statements
This prospectus and the documents incorporated by reference herein contain forward-looking statements that are based on management’s beliefs and assumptions and on information currently available to management. Some of the statements under “Prospectus summary,” “Risk factors,” and “Business” and elsewhere in this prospectus and the documents incorporated by reference herein (including “Management’s Discussion and Analysis of Financial Conditions and Results of Operations” from our 2013 Annual Report and our June 2014 Quarterly Report) contain forward-looking statements. In some cases, you can identify forward-looking statements by the following words: “may,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “ongoing” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words.
These statements involve risks, uncertainties and other factors that may cause actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. Although we believe that we have a reasonable basis for each forward-looking statement contained in this prospectus and the documents incorporated by reference herein, we caution you that these statements are based on a combination of facts and factors currently known by us and our projections of the future, about which we cannot be certain.
In addition, you should refer to the “Risk factors” section of this prospectus for a discussion of other important factors that may cause actual results to differ materially from those expressed or implied by the forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this prospectus will prove to be accurate. Furthermore, if the forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. The Private Securities Litigation Reform Act of 1995 and Section 27A of the Securities Act of 1933 do not protect any forward-looking statements that we make in connection with this offering.
This prospectus contains market data and industry forecasts that were obtained from industry publications. These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. We have not independently verified any third-party information. While we believe the market position, market opportunity and market size information included in this prospectus is generally reliable, such information is inherently imprecise.
-32-
The selling stockholders will receive all of the net proceeds from the sale of the shares offered hereby. We will not receive any proceeds from the sale of shares by the selling stockholders.
Our common stock began trading on the NASDAQ Global Select Market under the symbol “INGN” on February 14, 2014. Prior to that date, there was no public market for our common stock.
On October 10, 2014, the closing price per share of our common stock as reported on the NASDAQ Global Select Market was $20.45 per share.
As of September 30, 2014, there were approximately 34 holders of record of our common stock, which does not reflect holders who beneficially own our common stock held in nominee or street name or stockholders whose shares may be held in trust by other entities.
The price range per share of common stock presented below represents the highest and lowest sales prices of our common stock on the NASDAQ Global Select Market for each quarterly period since our initial public offering.
|
2014 |
|
High |
|
|
Low |
|
||
|
First Quarter ended March 31, 2014 (beginning February 14, 2014) |
|
$ |
20.9997 |
|
|
$ |
14.78 |
|
|
Second Quarter ended June 30, 2014 |
|
$ |
22.62 |
|
|
$ |
13.1201 |
|
|
Third Quarter ended September 30, 2014 |
|
$ |
24.50 |
|
|
$ |
17.72 |
|
|
Fourth Quarter ended December 31, 2014 (through October 10, 2014) |
|
$ |
21.92 |
|
|
$ |
20.25 |
|
-33-
We have never declared or paid any cash dividends on our common stock or any other securities. We anticipate that we will retain all available funds and any future earnings, if any, for use in the operation of our business and do not anticipate paying cash dividends in the foreseeable future. Payment of future cash dividends, if any, will be at the discretion of our board of directors after taking into account various factors, including our financial condition, operating results, current and anticipated cash needs, the requirements of current or then-existing debt instruments and other factors our board of directors deems relevant.
-34-
The following table sets forth our cash and cash equivalents and capitalization as of June 30, 2014.
You should read this information together with our financial statements and related notes and the information under the captions “Selected financial data” in this prospectus and “Management Discussion and Analysis of Financial Conditions and Results of Operations,” appearing in our 2013 Annual Report and in our June 2014 Quarterly Report, incorporated by reference in this prospectus.
|
|
|
As of June 30, 2014 |
|
|
|
(in thousands, except per share and share amounts) |
|
(unaudited) |
|
|
|
Cash and cash equivalents |
|
$ |
69,046 |
|
|
|
|
|
|
|
|
Long-term debt, including current maturities |
|
$ |
13,413 |
|
|
Stockholders’ equity (deficit): |
|
|
|
|
|
Preferred stock, $0.001 par value per share; 10,000,000 authorized, no shares issued or outstanding |
|
|
— |
|
|
Common stock, $0.001 par value per share, 200,000,000 shares authorized, 18,240,043 shares issued and outstanding |
|
|
18 |
|
|
Additional paid-in capital |
|
|
171,141 |
|
|
Accumulated deficit |
|
|
(60,345 |
) |
|
Total stockholders’ equity |
|
|
110,814 |
|
|
Total capitalization |
|
$ |
124,227 |
|
The outstanding share information in the table above excludes as of June 30, 2014:
|
• |
2,842,736 shares of common stock issuable upon the exercise of options to purchase common stock as of June 30, 2014, at a weighted average exercise price of $5.21 per share; |
|
• |
462,249 shares of common stock reserved for future grants under our stock-based compensation plans as of June 30, 2014 of this prospectus, consisting of: |
|
• |
283,180 shares of common stock reserved for future grants under our 2014 Equity Incentive Plan; and |
|
• |
179,069 shares of common stock reserved for future issuance under our 2014 Employee Stock Purchase Plan; and |
|
• |
Any shares of common stock that become available subsequent to this offering under our 2014 Equity Incentive Plan and 2014 Employee Stock Purchase Plan pursuant to the provisions thereof that automatically increase the shares reserved for issuance under such plans each year, as more fully described in “Employee benefit and stock plans;” and |
|
• |
161,295 shares of common stock issuable upon the exercise of warrants outstanding as of June 30, 2014, at a weighted average exercise price of $0.30 per share. |
-35-
You should read the following selected financial data below in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the financial statements, related notes and other financial information incorporated by reference in this prospectus from our 2013 Annual Report and our June 2014 Quarterly Report. The selected financial data in this section is not intended to replace the financial statements and is qualified in their entirety by the financial statements and related notes which are incorporated by reference in this prospectus.
The statements of operations data for the years ended December 31, 2013, 2012 and 2011 and the balance sheet data as of December 31, 2013, 2012 and 2011 are derived from our audited financial statements included in our 2013 Annual Report which is incorporated by reference in this prospectus. The statements of operations data for the six months ended June 30, 2014 and 2013 and the balance sheet data as of June 30, 2014 are derived from our unaudited interim financial statements included in our June 2014 Quarterly Report which is incorporated by reference in this prospectus. Our unaudited interim financial statements were prepared on a basis consistent with our audited financial statements and include, in our opinion, all adjustments, consisting only of normal recurring adjustments that we consider necessary for a fair presentation of the financial information set forth in those statements included in our June 2014 Quarterly Report. Our historical results are not necessarily indicative of the results that may be expected in any future period, and our interim results are not necessarily indicative of the results that may be expected for the full year or any other period.
|
|
Year ended December 31, |
|
|
Six months ended June 30, |
|
||||||||||||||
|
|
|
2013 |
|
|
|
2012 |
|
|
|
2011 |
|
|
|
2014 |
|
|
|
2013 |
|
|
(amounts in thousands, except share and per share amounts) |
|
|
|
|
|
|
|
|
|
|
|
|
(unaudited) |
|
|||||
|
Revenue |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales revenue |
$ |
43,971 |
|
|
$ |
28,077 |
|
|
$ |
19,076 |
|
|
$ |
35,321 |
|
|
$ |
21,646 |
|
|
Rental revenue |
|
30,538 |
|
|
|
19,872 |
|
|
|
10,977 |
|
|
|
18,705 |
|
|
|
14,258 |
|
|
Sales of used rental equipment revenue |
|
200 |
|
|
|
95 |
|
|
|
46 |
|
|
|
— |
|
|
|
— |
|
|
Other revenue |
|
734 |
|
|
|
532 |
|
|
|
535 |
|
|
|
— |
|
|
|
— |
|
|
Total revenue |
|
75,443 |
|
|
|
48,576 |
|
|
|
30,634 |
|
|
|
54,026 |
|
|
|
35,904 |
|
|
Cost of revenue |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of sales revenue |
|
24,209 |
|
|
|
17,359 |
|
|
|
12,127 |
|
|
|
18,223 |
|
|
|
11,655 |
|
|
Cost of rental revenue |
|
12,146 |
|
|
|
7,243 |
|
|
|
3,783 |
|
|
|
8,751 |
|
|
|
5,075 |
|
|
Cost of used rental equipment revenue |
|
97 |
|
|
|
25 |
|
|
|
20 |
|
|
|
— |
|
|
|
— |
|
|
Total cost of revenue |
|
36,452 |
|
|
|
24,627 |
|
|
|
15,930 |
|
|
|
26,974 |
|
|
|
16,730 |
|
|
Gross profit |
|
38,991 |
|
|
|
23,949 |
|
|
|
14,704 |
|
|
|
27,052 |
|
|
|
19,174 |
|
|
Operating expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
2,398 |
|
|
|
2,262 |
|
|
|
1,789 |
|
|
|
1,514 |
|
|
|
1,143 |
|
|
Sales and marketing |
|
18,375 |
|
|
|
12,569 |
|
|
|
9,014 |
|
|
|
12,069 |
|
|
|
8,742 |
|
|
General and administrative |
|
13,754 |
|
|
|
8,289 |
|
|
|
5,623 |
|
|
|
7,957 |
|
|
|
6,264 |
|
|
Total operating expenses |
|
34,527 |
|
|
|
23,120 |
|
|
|
16,426 |
|
|
|
21,540 |
|
|
|
16,149 |
|
|
Income (loss) from operations |
|
4,464 |
|
|
|
829 |
|
|
|
(1,722 |
) |
|
|
5,512 |
|
|
|
3,025 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total other expense, net |
|
(616 |
) |
|
|
(247 |
) |
|
|
(267 |
) |
|
|
(271 |
) |
|
|
(227 |
) |
|
Income (loss) before provision (benefit) for income taxes |
|
3,848 |
|
|
|
582 |
|
|
|
(1,989 |
) |
|
|
5,241 |
|
|
|
2,798 |
|
|
Provision (benefit) for income taxes |
|
(21,587 |
) |
|
|
18 |
|
|
|
13 |
|
|
|
2,067 |
|
|
|
108 |
|
|
Net income (loss) |
$ |
25,435 |
|
|
$ |
564 |
|
|
$ |
(2,002 |
) |
|
$ |
3,174 |
|
|
$ |
2,690 |
|
|
Less deemed dividend on redeemable convertible preferred stock |
|
(7,278 |
) |
|
|
(5,781 |
) |
|
|
(3,027 |
) |
|
|
(987 |
) |
|
|
(3,508 |
) |
|
Net income (loss) before preferred rights dividend |
$ |
18,157 |
|
|
$ |
(5,217 |
) |
|
$ |
(5,029 |
) |
|
$ |
2,187 |
|
|
$ |
(818 |
) |
|
Less preferred rights dividend on redeemable convertible preferred stock |
|
(7,165 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Less undistributed earnings to preferred stock |
|
(10,781 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Net income (loss) attributable to common stockholders |
$ |
211 |
|
|
$ |
(5,217 |
) |
|
$ |
(5,029 |
) |
|
$ |
2,187 |
|
|
$ |
(818 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic net income (loss) per share attributable to common stockholders |
$ |
0.76 |
|
|
$ |
(19.97 |
) |
|
$ |
(20.15 |
) |
|
$ |
0.13 |
|
|
$ |
(2.98 |
) |
|
Diluted net income (loss) per share attributable to common stockholders |
$ |
0.68 |
|
|
$ |
(19.97 |
) |
|
$ |
(20.15 |
) |
|
$ |
0.11 |
|
|
$ |
(2.98 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average number of shares used in calculating income (loss) per share attributable to common stockholders (note 1): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic common shares |
|
276,535 |
|
|
|
261,268 |
|
|
|
249,519 |
|
|
|
13,843,803 |
|
|
|
274,396 |
|
|
Diluted common shares |
|
2,008,156 |
|
|
|
261,268 |
|
|
|
249,519 |
|
|
|
15,826,754 |
|
|
|
274,396 |
|
|
(1) |
See note 2 to our 2013 Annual Report and to our interim financial statements included in our June 2014 Quarterly Report for an explanation of the calculations of our basic and diluted net income (loss) per share attributable to common stockholders. |
-36-
|
|
|
As of December 31, |
|
|
As of June 30, |
|
||||||||||||||
|
(amounts in thousands) |
|
2013 |
|
|
2012 |
|
|
2011 |
|
|
2014 |
|
|
2013 |
|
|||||
|
|
|
|
|
|
unaudited |
|
||||||||||||||
|
Balance sheet data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
Cash and cash equivalents |
|
$ |
13,521 |
|
|
$ |
15,112 |
|
|
$ |
3,906 |
|
|
$ |
69,046 |
|
|
$ |
3,906 |
|
|
Working capital |
|
|
13,159 |
|
|
|
12,880 |
|
|
|
1,302 |
|
|
|
71,394 |
|
|
|
13,548 |
|
|
Total assets |
|
|
82,397 |
|
|
|
47,586 |
|
|
|
24,131 |
|
|
|
145,466 |
|
|
|
82,397 |
|
|
Total indebtedness |
|
|
10,649 |
|
|
|
8,936 |
|
|
|
9,629 |
|
|
|
13,413 |
|
|
|
10,649 |
|
|
Deferred revenue |
|
|
2,263 |
|
|
|
1,094 |
|
|
|
594 |
|
|
|
3,530 |
|
|
|
2,263 |
|
|
Total liabilities |
|
|
26,098 |
|
|
|
19,011 |
|
|
|
16,575 |
|
|
|
34,652 |
|
|
|
26,098 |
|
|
Redeemable convertible preferred stock |
|
|
118,671 |
|
|
|
109,345 |
|
|
|
83,122 |
|
|
|
— |
|
|
|
118,671 |
|
|
Total stockholders’ (deficit) equity |
|
$ |
(62,372 |
) |
|
$ |
(80,770 |
) |
|
$ |
(75,566 |
) |
|
$ |
110,814 |
|
|
$ |
(62,372 |
) |
Non-GAAP financial measures
EBITDA, Adjusted EBITDA, and Adjusted net income (loss) are financial measures that are not calculated in accordance with generally accepted accounting principles in the United States, or GAAP. We define EBITDA as net income or loss excluding interest income, interest expense, taxes and depreciation and amortization. Adjusted EBITDA also excludes the change in the fair value of our preferred stock warrant liability and stock-based compensation. Below, we have provided a reconciliation of EBITDA, Adjusted EBITDA, and Adjusted net income (loss) to our net income or loss, the most directly comparable financial measure calculated and presented in accordance with GAAP. EBITDA, Adjusted EBITDA, and Adjusted net income (loss) should not be considered alternatives to net income or loss or any other measure of financial performance calculated and presented in accordance with GAAP. Our EBITDA, and Adjusted EBITDA, and Adjusted net income (loss) may not be comparable to similarly titled measures of other organizations because other organizations may not calculate EBITDA, Adjusted EBITDA, and Adjusted net income (loss) in the same manner as we calculate these measures.
We include EBITDA, Adjusted EBITDA and Adjusted net income (loss) in this prospectus because they are important measures upon which our management assesses our operating performance. We use EBITDA, Adjusted EBITDA and Adjusted net income (loss) as key performance measures because we believe they facilitate operating performance comparisons from period to period by excluding potential differences primarily caused by variations in capital structures, tax positions, the impact of depreciation and amortization expense on our fixed assets, changes related to the fair value re-measurements of our preferred stock warrant, and the impact of stock-based compensation expense. Because EBITDA, Adjusted EBITDA and Adjusted net income (loss) facilitate internal comparisons of our historical operating performance on a more consistent basis, we also use EBITDA, Adjusted EBITDA and Adjusted net income (loss) for business planning purposes, to incentivize and compensate our management personnel, and in evaluating acquisition opportunities. In addition, we believe EBITDA, Adjusted EBITDA and Adjusted net income (loss) and similar measures are widely used by investors, securities analysts, ratings agencies, and other parties in evaluating companies in our industry as a measure of financial performance and debt-service capabilities.
Our use of EBITDA, Adjusted EBITDA and Adjusted net income (loss) have limitations as analytical tools, and you should not consider them in isolation or as a substitute for analysis of our results as reported under GAAP. Some of these limitations are:
|
• |
EBITDA, Adjusted EBITDA and Adjusted net income (loss) do not reflect our cash expenditures for capital equipment or other contractual commitments; |
|
• |
Although depreciation and amortization are non-cash charges, the assets being depreciated and amortized may have to be replaced in the future, and EBITDA, Adjusted EBITDA and Adjusted net income (loss) do not reflect capital expenditure requirements for such replacements; |
|
• |
EBITDA, Adjusted EBITDA and Adjusted net income (loss) do not reflect changes in, or cash requirements for, our working capital needs; |
|
• |
EBITDA, Adjusted EBITDA, and Adjusted net income (loss) do not reflect the interest expense or the cash requirements necessary to service interest or principal payments on our indebtedness; and |
|
• |
Other companies, including companies in our industry, may calculate EBITDA, Adjusted EBITDA and Adjusted net income (loss) measures differently, which reduces their usefulness as a comparative measure. |
-37-
In evaluating EBITDA, Adjusted EBITDA, and Adjusted net income (loss) you should be aware that in the future we will incur expenses similar to the adjustments in this presentation. Our presentation of EBITDA, Adjusted EBITDA and Adjusted net income (loss) should not be construed as an inference that our future results will be unaffected by these expenses or any unusual or non-recurring items. When evaluating our performance, you should consider EBITDA, Adjusted EBITDA and Adjusted net income (loss) alongside other financial performance measures, including our net loss and other GAAP results.
The following table presents a reconciliation of EBITDA, Adjusted EBITDA and Adjusted net income (loss) to our net income or loss, the most comparable GAAP measure, for each of the periods indicated:
|
(amounts in thousands) |
|
Year ended December 31, |
|
|
Six months ended June 30, |
|
||||||||||||||
|
EBITDA (1) |
|
2013 |
|
|
2012 |
|
|
2011 |
|
|
2014 |
|
|
2013 |
|
|||||
|
unaudited |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
25,435 |
|
|
$ |
564 |
|
|
$ |
(2,002 |
) |
|
$ |
3,174 |
|
|
$ |
2,690 |
|
|
Non-GAAP adjustments: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense |
|
|
562 |
|
|
|
493 |
|
|
|
261 |
|
|
|
336 |
|
|
|
199 |
|
|
Interest income |
|
|
(12 |
) |
|
|
(88 |
) |
|
|
(113 |
) |
|
|
(18 |
) |
|
|
(6 |
) |
|
Provision (benefit) for income taxes |
|
|
(21,587 |
) |
|
|
18 |
|
|
|
13 |
|
|
|
2,067 |
|
|
|
108 |
|
|
Depreciation and amortization |
|
|
8,544 |
|
|
|
4,984 |
|
|
|
3,198 |
|
|
|
5,586 |
|
|
|
3,653 |
|
|
EBITDA |
|
|
12,942 |
|
|
|
5,971 |
|
|
|
1,357 |
|
|
|
11,145 |
|
|
|
6,644 |
|
|
Change in fair value of preferred stock warrant liability |
|
|
262 |
|
|
|
(148 |
) |
|
|
119 |
|
|
|
(36 |
) |
|
|
243 |
|
|
Stock-based compensation |
|
|
230 |
|
|
|
60 |
|
|
|
144 |
|
|
|
666 |
|
|
|
51 |
|
|
Adjusted EBITDA |
|
$ |
13,434 |
|
|
$ |
5,883 |
|
|
$ |
1,620 |
|
|
$ |
11,775 |
|
|
$ |
6,938 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) (GAAP) |
|
$ |
25,435 |
|
|
$ |
564 |
|
|
$ |
(2,002 |
) |
|
$ |
3,174 |
|
|
$ |
2,690 |
|
|
One-time benefit from reversal of deferred tax valuation adjustment |
|
|
(21,807 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Adjusted net income (loss) |
|
$ |
3,628 |
|
|
$ |
564 |
|
|
$ |
(2,002 |
) |
|
$ |
3,174 |
|
|
$ |
2,690 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(amounts in thousands, except share and per share amounts) |
|
Year ended December 31, |
|
|
|
|
|
|
Six months ended June 30, |
|
||||||||||
|
Pro-forma non-GAAP results of EPS calculation (2)(3)(4) |
|
2013 |
|
|
2012 |
|
|
|
|
|
|
2014 |
|
|
2013 |
|
||||
|
Unaudited |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Numerator - basic and diluted: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income |
|
$ |
25,435 |
|
|
$ |
564 |
|
|
|
|
|
|
$ |
3,174 |
|
|
$ |
2,690 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pro-forma net income per share - basic common stock |
|
$ |
1.74 |
|
|
$ |
0.04 |
|
|
|
|
|
|
$ |
0.18 |
|
|
$ |
0.19 |
|
|
Pro-forma net income per share - diluted common stock |
|
$ |
1.55 |
|
|
$ |
0.04 |
|
|
|
|
|
|
$ |
0.16 |
|
|
$ |
0.17 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Denominator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pro-forma weighted-average common shares - basic common stock |
|
|
14,636,950 |
|
|
|
14,601,861 |
|
|
|
|
|
|
|
17,308,133 |
|
|
|
14,530,870 |
|
|
Pro-forma weighted-average common shares - diluted common stock |
|
|
16,368,571 |
|
|
|
15,486,487 |
|
|
|
|
|
|
|
19,291,084 |
|
|
|
15,654,526 |
|
|
(1) |
For a discussion of our use of EBITDA, Adjusted EBITDA, and Adjusted net income (loss) and their calculations, please see “—Non GAAP financial measures.” |
|
(2) |
The pro forma EPS calculations gives effect to: the automatic conversion of the outstanding convertible preferred stock into a weighted average of 14,219,001 and 14,216,838 shares of common stock, for the years ended December 31, 2013 and 2012, and 14, 219,001 and 14,057,509 for the six months ended June 30, 2014 and 2013. |
|
(3) |
The pro forma EPS calculations gives effect to: the cash exercise of warrants to purchase an aggregate of 142,495 shares of common stock, which was expected to occur prior to closing of our initial public offering as the warrants would have otherwise expired at that time for the years ended December 31, 2013 and 2012 and 130,385 for the six months ended June 30, 2013. |
|
(4) |
The pro forma EPS calculations gives effect to the reclassification of our preferred stock warrant liability to additional paid-in-capital upon the closing of our initial public offering. |
-38-
Quarterly financial data
The following tables set forth selected unaudited quarterly statements of operations data for the last ten fiscal quarters. The unaudited interim financial statements for each of these quarters have been prepared on the same basis as the audited financial statements included in our 2013 Annual Report and, in the opinion of management, reflect all adjustments, which include only normal recurring adjustments, necessary to a fair statement of our results of operations and financial position for these periods. These data should be read in conjunction with the audited financial statements and accompanying notes included in our 2013 Annual Report. The 2014 quarterly operating results are not necessarily indicative of our operating results for any future period.
The following tables present our selected quarterly financial for the last ten quarters.
|
|
|
2014 |
|
|
|
|
|
|
|
|
|
|||||
|
(amounts in thousands, except per share amounts) |
|
1st Qtr |
|
|
2nd Qtr |
|
|
|
|
|
|
|
|
|
||
|
unaudited |
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Quarterly Results |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net sales |
|
$ |
23,633 |
|
|
$ |
30,393 |
|
|
|
|
|
|
|
|
|
|
Gross profit |
|
|
11,938 |
|
|
|
15,114 |
|
|
|
|
|
|
|
|
|
|
Net income |
|
$ |
888 |
|
|
$ |
2,286 |
|
|
|
|
|
|
|
|
|
|
Net income (loss) per share attributable to common stockholders |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic - common stockholders |
|
|
(0.01 |
) |
|
|
0.13 |
|
|
|
|
|
|
|
|
|
|
Diluted - common stockholders |
|
|
(0.01 |
) |
|
|
0.11 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2013 |
|
|||||||||||||
|
(amounts in thousands, except per share amounts) |
|
1st Qtr |
|
|
2nd Qtr |
|
|
3rd Qtr |
|
|
4th Qtr |
|
||||
|
Unaudited |
|
|
|
|
|
|
|
|||||||||
|
Quarterly Results |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net sales |
|
$ |
15,747 |
|
|
$ |
20,157 |
|
|
$ |
19,777 |
|
|
$ |
19,762 |
|
|
Gross profit |
|
|
8,117 |
|
|
|
11,057 |
|
|
|
9,645 |
|
|
|
10,175 |
|
|
Net income |
|
$ |
730 |
|
|
$ |
1,960 |
|
|
$ |
774 |
|
|
$ |
21,971 |
|
|
Net income (loss) per share attributable to common stockholders |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic - common stockholders |
|
|
(3.65 |
) |
|
|
0.00 |
|
|
|
(3.90 |
) |
|
|
0.91 |
|
|
Diluted - common stockholders |
|
|
(3.65 |
) |
|
|
0.00 |
|
|
|
(3.90 |
) |
|
|
0.79 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2012 |
|
|||||||||||||
|
(amounts in thousands, except per share amounts) |
|
1st Qtr |
|
|
2nd Qtr |
|
|
3rd Qtr |
|
|
4th Qtr |
|
||||
|
Unaudited |
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Quarterly Results |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net sales |
|
$ |
9,244 |
|
|
$ |
12,340 |
|
|
$ |
13,151 |
|
|
$ |
13,841 |
|
|
Gross profit |
|
|
4,576 |
|
|
|
5,842 |
|
|
|
6,496 |
|
|
|
7,035 |
|
|
Net income (loss) |
|
$ |
(212 |
) |
|
$ |
446 |
|
|
$ |
222 |
|
|
$ |
108 |
|
|
Net loss per share attributable to common stockholders |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic - common stockholders |
|
|
(3.37 |
) |
|
|
(5.54 |
) |
|
|
(5.12 |
) |
|
|
(5.71 |
) |
|
Diluted - common stockholders |
|
|
(3.37 |
) |
|
|
(5.54 |
) |
|
|
(5.12 |
) |
|
|
(5.71 |
) |
Our quarterly revenue and results of operations have varied in the past and we expect them to continue to vary significantly from quarter-to-quarter. These fluctuations are due to numerous factors, including: fluctuations in consumer demand for our products; seasonal cycles in consumer spending; our ability to design, manufacture and deliver products to our consumers in a timely and cost-effective manner; quality control problems in our manufacturing operations; our ability to timely obtain adequate quantities of the components used in our products; new product introductions and enhancements by us and our competitors; unanticipated increases in costs or expenses; and fluctuations in foreign currency exchange rates. We typically experience higher sales in the second quarter, as a result of consumers traveling and vacationing during the summer months.
-39-
Overview
We are a medical technology company that develops, manufactures and markets innovative portable oxygen concentrators used to deliver supplemental long-term oxygen therapy to patients suffering from chronic respiratory conditions. Traditionally, these patients have relied on stationary oxygen concentrator systems for use in the home and oxygen tanks or cylinders for mobile use, which we call the delivery model. The tanks and cylinders must be delivered regularly and have a finite amount of oxygen, which requires patients to plan activities outside of their homes around delivery schedules and a finite oxygen supply. Additionally, patients must attach long, cumbersome tubing to their stationary concentrators simply to enable mobility within their homes. Our proprietary Inogen One systems concentrate the air around the patient to offer a single source of supplemental oxygen anytime, anywhere with a portable device weighing approximately 4.8 or 7.0 pounds. Our Inogen One G3 and G2 have up to 4.5 and 5 hours of battery life, respectively, with a single battery and can be plugged into an outlet when at home, in a car, or in a public place with outlets available. Our systems reduce the patient’s reliance on stationary concentrators and scheduled deliveries of tanks with a finite supply of oxygen, thereby improving patient quality of life and fostering mobility.
Although portable oxygen concentrators represent the fastest-growing segment of the Medicare oxygen therapy market, we estimate based on 2012 Medicare data that patients using portable oxygen concentrators represent approximately 4% to 5% of the total addressable oxygen market in the United States. Based on 2012 industry data, we were the leading worldwide manufacturer of portable oxygen concentrators, as well as the largest provider of portable oxygen concentrators to Medicare patients, as measured by dollar volume. We believe we are the only manufacturer of portable oxygen concentrators that employs a direct-to-consumer strategy in the United States, meaning we market our products to patients, process their physician paperwork, provide clinical support as needed and bill Medicare or insurance on their behalf. To pursue a direct-to-consumer strategy, our manufacturing competitors would need to meet national accreditation and state-by-state licensing requirements and secure Medicare billing privileges, as well as compete with the home medical equipment providers that many rely on across their entire homecare business.
We believe our direct-to-consumer strategy has been critical to driving patient adoption of our technology. Other portable oxygen concentrator manufacturers access patients by selling through home medical equipment providers that we believe are disincentivized to encourage adoption of portable oxygen concentrators. In order to facilitate the regular delivery and pickup of oxygen tanks, home medical equipment providers have invested in geographically dispersed distribution infrastructure consisting of delivery vehicles, physical locations and delivery personnel within each area. Because portable oxygen concentrators eliminate the need for a physical distribution infrastructure, but have higher initial equipment costs than the delivery model, we believe converting to a portable oxygen concentrator model would require significant restructuring and capital investment for home medical equipment providers. Our direct-to-consumer marketing strategy allows us to sidestep the home medical equipment channel, appeal to patients directly and capture both the manufacturing and provider margin associated with long-term oxygen therapy. We believe our ability to capture this top-to-bottom margin, combined with our portable oxygen concentrators technology that eliminates the need for the service and infrastructure costs associated with the delivery model, gives us a cost structure advantage over our competitors.
Since adopting our direct-to-consumer strategy in 2009 following our acquisition of Comfort Life Medical Supply, LLC, which had an active Medicare billing number but few other assets and limited business activities, we have directly sold or rented our Inogen One systems to more than 40,000 patients, growing our revenue from $10.7 million in 2009 to $75.4 million in 2013. In 2013, 22.2% of our revenue came from our international markets and 40.5% of our revenue came from oxygen rentals. Our percentage of rental revenue increased from 35.8% in 2011, increasing our proportion of recurring revenue. Additionally, we have increased our gross margin from 49.3% in 2012 to 51.7% in 2013 primarily due to the change in sales mix toward direct-to-consumer from provider sales, improving system reliability, reducing material cost per system and lowering overhead cost per system. Our net loss was $2.6 million in 2009 transitioning to net income of $25.4 million in 2013. Adjusted net income excluding a one-time tax benefit was $3.6 million in 2013.
-40-
Our market
Overview of oxygen therapy market
We believe the current total addressable oxygen therapy market in the United States is approximately $3 billion to $4 billion, based on 2012 Medicare data and our estimate of the ratio of the Medicare market to the total market. We estimate that more than 2.5 million patients in the United States and more than 4.5 million patients worldwide use oxygen therapy, and more than 60% of oxygen therapy patients in the United States are covered by Medicare. The number of oxygen therapy patients in the United States is projected to grow by approximately 7% to 10% per year between 2013 and 2019, which we believe is the result of earlier diagnosis of chronic respiratory conditions, demographic trends and longer durations of long-term oxygen therapy.
Long-term oxygen therapy has been shown to be a cost-efficient and clinically effective means to treat hypoxemia, a condition in which patients have insufficient oxygen in the blood. Hypoxemic patients are unable to convert oxygen found in the air into the bloodstream in an efficient manner, causing organ damage and poor health. Chronic obstructive pulmonary disease, or COPD, is a leading cause of hypoxemia. Approximately 70% of our patient population has been diagnosed with COPD, which we believe is reflective of the long-term oxygen therapy market in general. Industry sources estimate that 24 million people in the United States suffer from COPD, of which one-half are undiagnosed.
According to our analysis of 2011 and 2012 Medicare data, approximately two-thirds of U.S. oxygen users require ambulatory oxygen and the remaining one-third require only stationary or nocturnal oxygen. Clinical data has shown that ambulatory patients that use oxygen twenty-four hours a day, seven days a week, or 24/7, regardless of whether such patients rely on portable oxygen concentrators or the delivery model, have approximately two times the survival rate and spend at least 60% fewer days annually in the hospital than non-ambulatory 24/7 patients. Of the ambulatory patients, we estimate that approximately 85% rely upon the delivery model that has the following disadvantages:
|
• |
limited flexibility outside the home, dictated by the finite oxygen supply provided by tanks and cylinders and dependence on delivery schedules; |
|
• |
restricted mobility and inconvenience within the home, as patients must attach long, cumbersome tubing to a noisy stationary concentrator to move within their homes; |
|
• |
products are not cleared for use on commercial aircraft and cannot plug into a vehicle outlet for extended use; and |
|
• |
high costs driven by the infrastructure necessary to establish a geographically diverse distribution network to serve patients locally, as well as personnel, fuel and other costs, which have limited economies of scale and generally increase over time. |
Portable oxygen concentrators were developed in response to many of the limitations associated with traditional oxygen therapy. Portable oxygen concentrators are designed to offer a self-replenishing, unlimited supply of oxygen that is concentrated from the surrounding air and to operate without the need for oxygen tanks or regular oxygen deliveries, allowing patients to enhance their independence and mobility. Additionally, because portable oxygen concentrators do not require the physical infrastructure and service intensity of the delivery model, we believe portable oxygen concentrators can provide long-term oxygen therapy with a lower cost structure. Despite the ability of portable oxygen concentrators to address many of the shortcomings of traditional oxygen therapy, we estimate based on 2012 Medicare data that the amount spent by patients with portable oxygen concentrators represents approximately 5% to 6% of total oxygen therapy spend. We believe the following has hindered the market acceptance of portable oxygen concentrators:
|
• |
to obtain portable oxygen concentrators, patients are dependent on home medical equipment providers, which have made significant investments in the physical distribution infrastructure to support the delivery model; |
|
• |
constrained manufacturing costs of conventional portable oxygen concentrators, driven by home medical equipment provider preference for products that have lower upfront equipment cost; and |
|
• |
limitations of conventional portable oxygen concentrators, including bulkiness, poor reliability and lack of suitability beyond intermittent or travel use. |
-41-
Our solution
Our Inogen One systems provide patients who require long-term oxygen therapy with a reliable, lightweight single solution product that improves quality of life, fosters mobility, and eliminates dependence on both oxygen tanks and oxygen cylinders as well as stationary concentrators. We believe our direct-to-consumer strategy increases our ability to effectively develop, design and market our Inogen One solutions, as it allows us to:
|
• |
drive patient awareness of our portable oxygen concentrator through direct marketing, sidestepping the home medical equipment channel that other manufacturers rely upon across their homecare businesses, and that is incentivized to continue to service oxygen patients through the delivery model; |
|
• |
capture the manufacturer and home medical equipment provider margins, allowing us to focus on the total cost of the solution and to invest in the development of product features instead of being constrained by the price required to attract representation from a distribution channel. For example, we have invested in features that improve patient satisfaction, product durability, reliability and longevity, which increase the cost of our hardware, but reduce the total cost of our solution by reducing our maintenance and repair cost; and |
|
• |
access and utilize direct patient feedback in our research and development efforts, allowing us to innovate based on this feedback and stay at the forefront of patient preference. For example, we have integrated a double battery into our product offering based on direct patient feedback. |
We believe the combination of our direct-to-consumer strategy with our singular focus on designing and developing oxygen concentrator technology has created the best-in-class portfolio of portable oxygen concentrators. Our two current product offerings, the Inogen One G3 and Inogen One G2, at approximately 4.8 and 7.0 pounds, respectively, are amongst the most lightweight portable oxygen concentrators on the market. We believe our Inogen One solutions offer the following benefits:
|
• |
Single solution for home, ambulatory, travel and nocturnal treatment. We believe our Inogen One solutions are the only portable oxygen concentrators marketed as a single solution, by which we mean a patient can use our Inogen One systems as their only supplemental oxygen source with no need to also use a stationary concentrator regularly. Our compressors are specifically designed to enable our patients to run our portable oxygen concentrators 24/7, whether powered by battery or plugged into an outlet at home or in a car while the battery is recharging. |
|
• |
Reliability. We have made product performance a priority and have improved reliability with each generation. For example, we have introduced patented air-dryer and patent-pending user-replaceable sieve beds to our products, which have improved product performance and, as a result, patient satisfaction. Reliability is not only critical to patient satisfaction, but also cost management, as our minimal physical infrastructure makes product exchanges more costly to us than providers with greater local physical infrastructure. |
|
• |
Effective for nocturnal use. Our Intelligent Delivery Technology enables our portable oxygen concentrators to provide consistent levels of oxygen during sleep despite decreased respiratory rates. As a result, patients can rely on the Inogen One G3 and Inogen One G2 portable oxygen concentrators overnight while sleeping. |
|
• |
Unparalleled flow capacity. Our 4.8 pound Inogen One G3 has at least 50% more flow capacity than other sub-5 pound portable oxygen concentrators, and our 7.0 pound Inogen One G2 has at least 15% more flow capacity than other sub-10 pound portable oxygen concentrators. |
|
• |
User friendly features. Our systems are designed with multiple user friendly features, including long battery life and low noise-levels in their respective weight categories. |
Our strengths
We believe our products and business model position us well to compete not only against other oxygen device manufacturers, but also to increase our share of the overall oxygen therapy market. We believe we have the following advantages relative to both traditional oxygen therapy providers and other oxygen device manufacturers:
|
• |
Attractive economic model. Our non-delivery model allows us to receive a premium monthly Medicare reimbursement for deployment of our devices to oxygen patients versus the delivery model. Standard Medicare reimbursement for ambulatory patients using the delivery model is $208.21 per month versus $229.87 per month for our portable oxygen concentrator model, representing a premium of $21.66 per month. A similar premium was maintained in the round one recompete ($19.09 per month) and in the round two ($23.30 per month) competitive bidding areas. In addition, we believe our portable oxygen concentrator technology and direct-to-consumer strategy allow us to provide our solutions through a more efficient cost structure. The delivery model requires ongoing gaseous or liquid oxygen container refills and regular home deliveries with accompanying costs, while our portable oxygen concentrator non-delivery model eliminates oxygen container refills and regular deliveries of oxygen containers and their associated costs. Following the first two rounds of competitive bidding and the round one recompete, we |
-42-
|
retained access to approximately 90% of the U.S. long-term oxygen therapy market, with the majority of contracts through mid-2016, while many providers were priced out of this market. |
|
• |
Direct-to-consumer capabilities. We believe our direct-to-consumer strategy enables patient access and retention as well as innovation and investment in our product portfolio. Pursuing a direct-to-consumer strategy requires national accreditation, state-by-state licensing and Medicare billing privileges. Given that we are unaware of any manufacturing competitor that currently markets on a direct-to-consumer basis, we do not believe any of these manufacturers possesses the necessary qualification to do so. If any of our manufacturing competitors were to pursue a direct-to-consumer strategy, they would risk negative reaction from the home medical equipment providers that sell their other homecare products, which generally represent significantly larger portions of their businesses than oxygen therapy products. |
|
• |
Commitment to customer service. We are focused on providing our patients with the highest quality of customer service. We guide them through the reimbursement and physician paperwork process, perform clinical titration and offer 24/7 telephone support, which includes clinical support as required. We have a sustained patient satisfaction rating of approximately 95%, as measured by our customer satisfaction surveys. |
|
• |
Patient-friendly, single-solution, sub-5 and sub-10 pound portable oxygen concentrators. Our Inogen One G3 and Inogen One G2 portable oxygen concentrators are sub-5 and sub-10 pound portable oxygen concentrators that can operate reliably and cost-effectively to service long-term oxygen therapy patients on a 24/7 basis, similar to a stationary oxygen concentrator or replacement portable oxygen concentrators. We believe the technology in our Inogen One portable oxygen concentrators is effective for nocturnal use, allowing patients to receive oxygen therapy around the clock from a single device. |
|
• |
Commitment to research and development and developing intellectual property portfolio. We have a broad patent portfolio covering the design and construction of our oxygen concentrators and system optimization. Additionally, we have made significant investments in research and development and have a robust product pipeline of next-generation oxygen concentrators. |
|
• |
Management team with proven track record and cost focus. Our management team has built our direct-to-consumer capabilities and launched our two current primary product offerings, Inogen One G2 and Inogen One G3. We continue to realize meaningful product manufacturing cost savings of approximately 40% from 2009 to 2013 as a result of management’s improvements in design, sourcing and reliability, as well as higher production volumes. |
|
• |
Revenue growth, profitability and recurring revenue. We have grown our revenue from $10.7 million in 2009 to $75.4 million in 2013, representing a continuous annual growth rate of 47.8%. In 2013, our recurring rental revenue represented 40.5% of sales. Our net income was $25.4 million after a one-time tax benefit of $21.8 million, or $3.6 million before the $21.8 million benefit compared to a net loss of $2.6 million in 2009. |
Our strategy
Our goal is to design, build and market oxygen solutions that redefine how oxygen therapy is delivered. To accomplish this goal, we will continue to invest in our product offerings and our commercial infrastructure to:
|
• |
expand our sales and marketing channels, including more internal and physician-based salespeople, increased direct-to-consumer advertising and greater international distribution; |
|
• |
develop innovative products, including next-generation oxygen concentrators and other innovations that improve quality of life; |
|
• |
secure contracts with private payors and Medicaid in order to become in-network with non-Medicare payors, which represent at least 30% of our home oxygen therapy patients, and we believe represent a younger and more active patient population; and |
|
• |
continue to focus on cost reduction through scalable manufacturing, reliability improvements, asset utilization and service cost reduction. |
-43-
Our Inogen One systems
We market our current product offerings, the Inogen One G3 and the Inogen One G2, as single solutions for oxygen therapy. This means our solutions can operate on a 24/7 basis for at least 60 months without a stationary concentrator. We believe the technology in our Inogen One G3 and our Inogen One G2 is effective for nocturnal use. Our Inogen One G2 and the Inogen One G3 are sub-5 and sub-10 pound portable oxygen concentrators that can operate reliably and cost-effectively over the long period of time needed to service oxygen therapy patients without supplemental use of a stationary concentrator or a replacement portable oxygen concentrator. To the extent our competitors’ portable oxygen solutions require supplemental use of a stationary oxygen concentrator, their solutions are less cost-effective and less convenient for patients. The following table summarizes our key product features:
|
|
Key Product Specifications
|
|
|
|
Inogen One G3
|
Inogen One G2
|
|
Capacity (ml/min) |
840 |
1,260 |
|
Weight (lbs) |
4.8 (single battery) |
7.0 (single battery) |
|
Battery run-time |
Up to 4.5 hours (single battery) |
Up to 5 hours (single battery) |
|
Maintenance prevention advantages |
User replaceable oxygen filtration |
Air dryer & user replaceable battery |
|
Technology effective for overnight use |
Yes |
Yes |
|
Sound |
42 dBA |
38 dBA |
We have focused our research and development efforts on creating solutions that we believe have overcome the reputation of portable oxygen concentrators as being limited in durability and reliability as well as unsuitable for nighttime or 24/7 use. We specifically designed our compressors for 24/7 use. We have worked to improve our reliability and reduce service costs by equipping our portable oxygen concentrators with features such as membrane air dryers and user replaceable filtration cartridges.
All of our Inogen One systems are equipped with Intelligent Delivery Technology, a form of pulse-dose technology from which the patient receives a bolus of oxygen upon inhalation. Pulse dose technology was developed to extend the number of hours an oxygen tank would last and is generally used on all ambulatory oxygen therapy devices. Our proprietary conserver technology utilizes differentiated triggering sensitivity to quickly detect a breath and ensure oxygen delivery within the first 400 milliseconds of inspiration, the interval when oxygen has the most effect on lung gas exchange. During periods of sleep, respiratory rates typically decrease. Our Inogen One systems actively respond to this changing physiology through the use of proprietary technology that increases bolus size. Our Intelligent Delivery Technology is designed to provide effective levels of blood oxygen saturation during sleep and all other periods of rest and activity that are substantially equivalent to continuous flow systems.
The Inogen One G3, our latest-generation portable oxygen concentrator, is among the most lightweight products on the market with substantially higher oxygen production capabilities than the other sub-5 pound portable oxygen concentrators on the market. We believe the performance parameters around the Inogen One G3 and Inogen One G2 allow us to serve approximately 95% of the ambulatory oxygen patients and enable us to address a patient’s particular clinical needs, as well as lifestyle and performance preferences.
Our direct-to-consumer business model has enabled us to receive direct patient feedback, and we have used this feedback to create portable oxygen concentrators that address the full suite of features and benefits critical to patient preference and retention. Our products prevent patients from having to choose between lightweight size, suitability for 24/7 use, reliability, and key features such as battery life, flow and reduced noise levels.
Sales and marketing
Our direct-to-consumer sales and marketing efforts are focused on generating awareness and demand for our Inogen One systems among patients, physicians and other clinicians, and third-party payors. In the United States as of December 31, 2013 we employed a marketing team of 5 people, an in-house sales team of 120 people, and a field-based sales force of 15 people. Of the $59 million of our 2013 revenue derived from the United States, approximately 52% represented direct-to-patient rentals, 30% represented cash pay sales to patients and 18% represented sales to third-party home medical equipment providers.
Our Medicare and private insurance patients rent our systems, while a portion of our patients choose to purchase our Inogen One products directly. Our ability to rent to patients directly, bill third-party payors on their behalf, and service patients in their homes requires that we hold a valid Medicare supplier number, are accredited by an independent agency approved by Medicare, and comply with the unique licensure and process requirements in the 49 states in which we serve patients.
-44-
We use a variety of direct-to-consumer marketing strategies to generate interest in our solutions among current oxygen therapy patients. After a patient contacts us, we guide them through product selection and insurance eligibility, and, if they choose to move forward, process the necessary reimbursement and physician paperwork on their behalf, as well as coordinate the shipping, instruction, and clinical setup process. In accordance with Medicare regulations we do not initially contact patients directly and contact them only upon an inbound inquiry. The below chart describes our United States direct-to-consumer sales process.
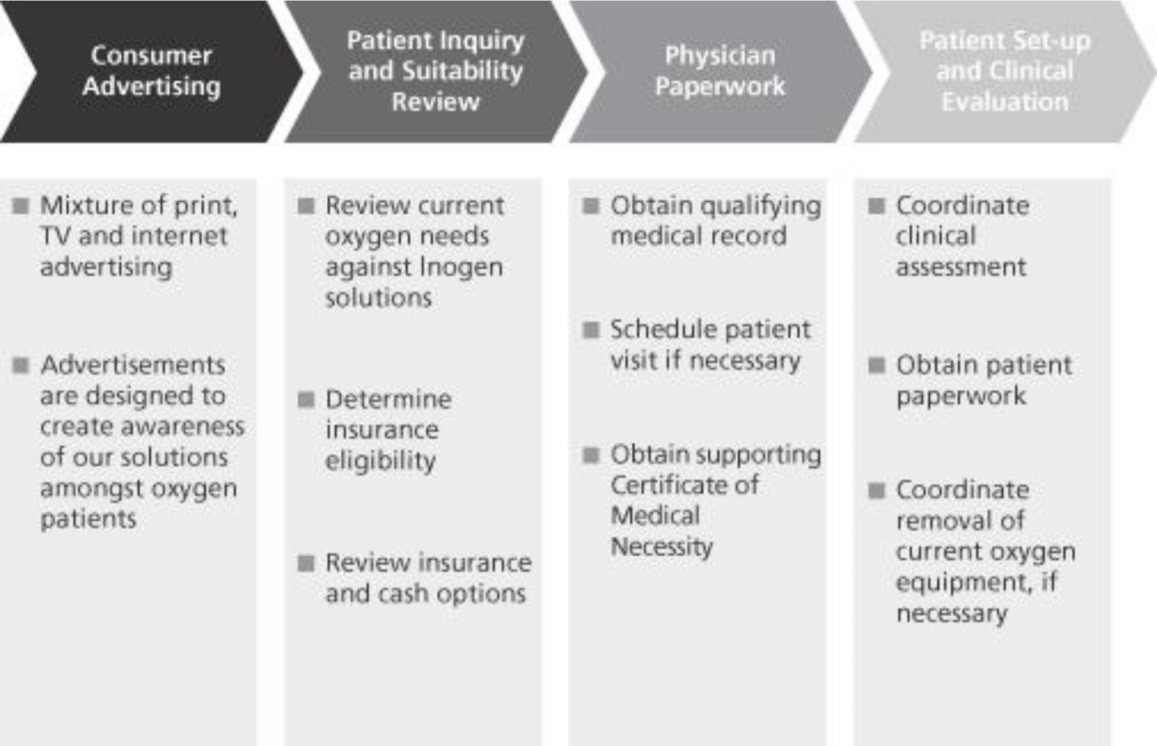
In addition to the direct-to-consumer sales model, we are increasingly utilizing a physician referral model as a complementary sales method. Under this model, our field sales representatives work with physicians in the representative’s territory to help physicians understand our products and the value these products provide for patients. We believe that by educating physicians on our products, we can cost-effectively supplement our direct-to-consumer sales and capture a greater number of patients earlier in the course of their oxygen therapy.
We engage in a number of other initiatives to increase awareness, demand, and orders for Inogen One systems. These include attendance at oxygen therapy support groups, guest speaking arrangements at trade shows, and product demonstrations as requested. Additionally, we are targeting private payors to become an in-network provider of oxygen therapy solutions, which we expect will reduce or eliminate any additional patient co-insurance associated with using our solution. We believe this will result in both increased conversion of our initial leads, as well as direct referrals from insurance companies in some cases.
International
Approximately 22% of our sales were from outside the United States in 2013. As of June 30, 2014, we sold our products in 44 countries outside the United States through distributors or directly to large “house” accounts, which include gas companies and home oxygen providers. In this case, we sell to and bill the distributor or “house” accounts directly, leaving the patient billing, support, and clinical setup to the local provider. As of December 31, 2013, we had four people who focused on selling our products to distributors and “house” accounts worldwide. In fiscal year 2012, an international distributor accounted for 12% of our revenue. However, no single international customer represented more than 10% of our total revenue for 2013, or for the six months ended June 30, 2014.
International sales have been a rapidly growing portion of our business, and we estimate there are 2 million long-term oxygen therapy patients outside of the United States. We believe that the international market is attractive for the following reasons:
|
• |
More favorable reimbursement in certain countries, including France and the United Kingdom, where portable oxygen concentrators receive more favorable reimbursement than in the United States. |
|
• |
Less developed oxygen delivery infrastructure in some countries. We believe that some countries outside the United States have less developed oxygen delivery infrastructure than in the United States. As a result, portable oxygen concentrators enable providers to reach and service patients they cannot economically reach with the delivery model. |
-45-
|
• |
An absence of reimbursement for any ambulatory oxygen therapy modalities in some countries, resulting in patients bearing all of the cost of ambulatory oxygen therapy and therefore becoming more involved in the selection of the modality. In Australia, for example, patients shoulder the burden of all costs associated with ambulatory oxygen therapy. In these cases, they tend to choose products like portable oxygen concentrators that provide a higher level of personal freedom. |
We will continue to focus on building out our international sales efforts.
Customer support and order fulfillment
Our procedures enable us to package and ship a system directly to the patient in the patient’s preferred configuration the same day the order is received. This enables us to minimize the amount of finished goods inventory we keep on hand. Our primary logistics partner is United Parcel Service, or UPS. UPS supports both our domestic and international shipments and provides additional services that support our direct-to-consumer oxygen therapy program. The UPS pick up service is used to retrieve patient paperwork, products requiring repair and systems that are no longer needed by the patient. Additionally, UPS, when necessary and requested by us, will go into a patient’s home to remove a replacement product from the box, box the failed device and return it to us. In this manner, we are able to operate as a remote provider while maintaining the level of customer service of a local oxygen therapy provider.
We believe it is crucial to provide patients with the highest quality customer support to achieve satisfaction with our products and optimal outcomes. As of December 31, 2013, we had a dedicated client service team of 24 people who were trained on our products, a clinical support team of 16 people who were licensed nurses or respiratory therapists, and a dedicated billing services team of 50 people. We provide our patients with a dedicated 24/7 hotline that is only given to our Inogen One patients and is not published publicly. Via the hotline, patients have direct access to our client services representatives, who can handle product-related questions. Additionally, clinical staff is on call 24/7 and available to patients whenever either the patient or the client services representative deems appropriate. Our dedicated billing services team is available to answer patient questions regarding invoicing, reimbursement, and account status during normal business hours. We receive no additional reimbursement for patient support, but provide high-quality customer service to enhance patient comfort, satisfaction, compliance, and safety with our products. We believe our focus on providing the highest level of customer service has helped drive our sustained patient satisfaction rating of approximately 95%.
Third-party reimbursement
Medicare or private insurance rentals represented approximately 40.5% of our revenue in 2013. In cases where we rent our oxygen therapy solutions directly to patients, we bill third-party payors, such as Medicare or private insurance, for monthly rentals on behalf of our patients. We process and coordinate all physician paperwork necessary for reimbursement of our solutions. A common medical criterion for oxygen therapy reimbursement is insufficient blood oxygen saturation level. Our team in sales and sales administration are trained on how to verify benefits, review medical records and process physician paperwork. Additionally, an independent internal review is performed and our products are not deployed until after physician paperwork is processed and reimbursement eligibility is verified and communicated to the patient. As of December 31, 2013, our sales and sales administration consisted of 135 people.
We are authorized by Medicare to bill for oxygen therapy, and we believe that more than 60% of oxygen therapy patients have Medicare coverage. Our Inogen One systems are reimbursed under HCPCS codes E1390 and E1392. E1390 covers stationary/nocturnal oxygen therapy systems, while E1392 provides additional reimbursement for portable oxygen concentrators for the treatment of ambulatory patients. Even though E1390 is a stationary oxygen code, we bill under both the E1390 and E1392 codes for our portable oxygen concentrators, assuming that the patient qualifies for portable oxygen, as well as stationary oxygen. Only in the event the patient solely qualifies for portable oxygen would we exclusively bill under the E1392 code, which is not typical. Currently, Medicare reimburses oxygen therapy as a monthly rental for up to 36 months. We retain equipment ownership at all times. After 36 months of payments, payment is “capped,” meaning the monthly payment amounts are discontinued. After two additional years or another qualifying event, the patient is eligible for replacement equipment and a new capped rental period.
As of January 1, 2011, Medicare has phased in a program called competitive bidding. Competitive bidding impacts the amount Medicare pays suppliers for durable medical equipment, including portable oxygen concentrators. The program is defined geographically, with suppliers submitting bids to provide medical equipment for a specific product category within that geography. Once bids have been placed, an individual company’s bids across products within the category are aggregated and weighted by each product’s market share in the category. The weighted average price is then indexed against competitors. Medicare determines a “clearing price” out of these weighted average prices at which sufficient suppliers have indicated they will support patients in the category, and this threshold is typically designed to have theoretical supply two times greater than expected demand. Bids for each modality among the suppliers that made the cut are then arrayed to determine what Medicare will reimburse for each product category. The program has strict anti-collusion guidelines to ensure bidding is truly competitive. Competitive bidding contracts last three years once implemented, after which they are subject to re-bidding or competitive bidding re-compete.
-46-
The competitive bidding program effectively reduces the number of oxygen suppliers that can participate in the Medicare program. We believe that more than 75% of existing oxygen suppliers were eliminated in round one of competitive bidding implemented January 1, 2011 in 9 U.S. Metropolitan Statistical Areas. Round two of competitive bidding was implemented July 1, 2013 in 91 U.S. Metropolitan Statistical Areas and we believe the impact on the number of oxygen suppliers will be similar when released. Combined with the round one of competitive bidding, we believe that approximately 59% of the market was covered by round one and two. The following table sets forth the current standard Medicare reimbursement rates and the weighted average of reimbursement rates applicable in Metropolitan Statistical Areas covered by rounds one and two of competitive bidding. The round one re-compete was completed in the same Metropolitan Statistical Areas as round one for the next three year period starting January 1, 2014 when the original contracts expire.
|
|
Medicare
|
Round one
|
Round two
|
Round one
|
|||||||
|
E1390 |
$ |
178.24 |
$ |
116.16 |
|
$ |
93.07 |
|
$ |
95.74 |
|
|
E1392 |
|
51.63 |
|
41.89 |
|
|
42.72 |
|
|
38.08 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
$ |
229.87 |
$ |
158.05 |
|
$ |
135.79 |
|
$ |
133.82 |
|
|
% of standard |
|
|
|
69 |
% |
|
59 |
% |
|
58 |
% |
Medicare has not announced specific plans to implement competitive bidding nationwide, but by 2016 Medicare must implement competitive bidding or competitive bidding pricing for included items in non-competitive bidding areas. In February 2014, Medicare solicited public comment on the methodology it would use to comply with this statute.
In July 2014, the Centers for Medicare and Medicaid services released a proposed rule that sets forth methodologies to adjust the fee schedule amounts for items subject to competitive bidding in areas where competitive bidding has not been implemented. The proposed rule applies rate reductions to all un-bid areas instead of doing an additional bidding process. The fee schedules in the un-bid areas would be adjusted based on regional averages of the single payment amounts for areas already under competitive bidding. The regional prices would be limited by a national ceiling (110% of the average of the regional prices) and a floor (90% of the average regional prices). The regions would be defined as follows:
|
Region Name |
|
States Covered |
|
Far West |
|
CA, NV, OR, WA |
|
Great Lakes |
|
IL, IN, MI, OH, WI |
|
Mideast |
|
DC, DE, MD, NJ, NY, PA |
|
New England |
|
CT, MA, NH, RI |
|
Plains |
|
IA, KS, MN, MO, NE |
|
Rocky Mountain |
|
CO, ID, UT |
|
Southeast |
|
AL, AR, FL, GA, KY, LA, NC, SC, TN, VA |
|
Southwest |
|
AZ, NM, OK, TX |
The Centers for Medicare and Medicaid proposes to define frontier states as state where more than 50% of the counties in the state have a population density of 6 people or less per square mile, and rural states are defined as a state where more than 50% of the population lives in rural areas per census data. Current frontier states include MT, ND, SD and WY; rural states include ME, MS, VT and WV; and non-contiguous United States areas include AK, HI, Guam and Puerto Rico. For frontier and rural states, the single payment amount would be the national ceiling (110% of the average of the regional prices) to account for higher servicing costs in these areas. For non-contiguous United States-areas, single payment amounts would be the higher of the national ceiling, or the average of competitive bidding pricing from these areas, if the areas had been bid through competitive bidding.
In addition, the Centers for Medicare and Medicaid Services is proposing to phase in bundled monthly payments for oxygen in no more than 12 CBAs, removing the 36 month cap on oxygen equipment payments, removing the separate add-on payments for non-delivery portable equipment and removing the separate payments for oxygen content. The payment would be bundled under one monthly rental fee for all rented equipment and related accessories, maintenance and servicing of the equipment and delivery of oxygen contents, and pricing would be determined through an additional competitive bidding round. The Centers for Medicare and Medicaid Services noted in the proposed rule that the 36 month cap is difficult to administer and few patients receive benefits in the capped period, noting that only 25% of the patients reach the end of the 36 month period. In this proposal, the current premium reimbursement for oxygen generating portable equipment, including our portable oxygen concentrators, would be eliminated. We cannot predict the impact this proposed rule, if finalized would have on our business, financial conditions and results of operations.
-47-
The Centers for Medicare and Medicaid Services also announced in July 2014 the schedule for competitive bidding round two re-compete, associated with approximately 50% of the market with contracts set to expire June 30, 2016. The Centers for Medicare and Medicaid Services intends to announce the bidding schedule in fall 2014 and commence bidding in winter 2015. The Centers for Medicare and Medicaid Services updated the product categories and the competitive bidding areas. Respiratory equipment includes oxygen, oxygen equipment, continuous positive airway pressure devices, respiratory assist devices and related supplies and accessories, such that nebulizers, are now their own separate product category instead of being included in the respiratory equipment category. Round two re-compete is in the same geographic areas that were included in the original round two. However, as a result of the Office of Management and Budget’s updates to the original 91 round two metropolitan statistical areas, there are now 90 metropolitan statistical areas for round two re-compete. The round two re-compete competitive bidding areas have nearly the same ZIP codes as the round two competitive bidding areas, the associated changes in the zip codes since the competitive bidding was implemented are reflective in this round two re-compete. Also, competitive bidding areas that were located in multi-state metropolitan statistical areas were defined so that no competitive bidding area is included in more than one state.
While we are monitoring the development and implementation of this proposal, we believe that the net effect of the proposal would be an approximately 4-5% decrease in 2016 revenue should the proposal become effective in its current form. Additionally, we expect that the stationary oxygen and non-delivery ambulatory oxygen payment rates will continue to fluctuate and a large negative payment adjustment could adversely affect our business, financial conditions and results of operations.
As of June 30, 2014, we had contracts with 58 non-Medicare payors. These contracts enable us to become an in-network provider for these payors, which enables patients to use our systems at the same cost as other in-network solutions, including the delivery model. Based on our patient population, we believe non-Medicare payors represent at least 30% of all oxygen therapy patients. We believe that private payor reimbursement levels will generally be reset in accordance with Medicare reimbursement levels determined by competitive bidding.
We cannot predict the extent to which reimbursement for our products will be affected by competitive bidding or by initiatives to reduce costs for private payors. The unavailability of third-party coverage or inadequacy of reimbursement for our current or future products would adversely affect our business, financial conditions, and results of operations.
Manufacturing
We have been developing and refining the manufacturing of our Inogen One systems over the past ten years. While nearly all of our manufacturing and assembly process was originally outsourced, assembly of the manifold, compressor, sieve bed and concentrator is now conducted in-house in order to improve quality control and reduce cost. Additionally, we use lean manufacturing practices to maximize our manufacturing efficiency. Bringing manufacturing and assembly largely in-house, combined with our consistent focus on driving efficient manufacturing processes, has enabled us to reduce our cost of revenue per system by 40% between 2009 and 2013.
We rely on third party manufacturers to supply several components of our Inogen One systems. We typically enter into supply agreements for these components that specify quantity, quality requirements, and delivery terms, which, in certain cases, can be terminated by either party upon relatively short notice. We have elected to source certain key components from single sources of supply, including our batteries, bearings, carry bags, motors, pistons, valves, and molded plastic components. While alternative sources of supply are readily available for these components, we believe that maintaining a single-source of supply allows us to control production costs and inventory levels, and to manage component quality. In order to mitigate against the risks related to a single-source of supply, we qualify alternative suppliers and develop contingency plans for responding to disruptions. If any single-source supplier were no longer able to supply a component, we believe we would be able to promptly and cost-effectively switch to an alternative supplier without a significant disruption to our business and operations. We have adopted additional contingency plans to protect against an immediate disruption in supply of our battery and motor components, and any potential delay that may result from a switch to a new supplier. These contingency plans include our own inventory management, along with a requirement that each supplier maintains specified quantities of inventory in multiple locations, and our maintenance of back-up tooling that can easily be transferred to the new supplier. We believe that these contingency plans would limit any disruption to our business in the event of an immediate termination of either our battery or motor supply.
We currently manufacture in two leased buildings in Goleta, California and Richardson, Texas, which we have registered with the FDA and for which we have obtained ISO 13485 certification. The Goleta, California facility is approximately 39,000 square feet. The Richardson, Texas facility is approximately 31,000 square feet. Because we have two separate manufacturing facilities, in the event one facility is incapacitated, the other facility will enable us to continue manufacturing our products to meet our current level of demand. We believe we have sufficient capacity to meet anticipated demand.
-48-
Our entire organization is responsible for quality management. Our Quality Assurance department oversees this by tracking component, device and organization performance and by training team members outside the Quality Assurance department to become competent users of our Quality Management system. By measuring component performance, communicating daily with the production group and our suppliers, and reviewing customer complaints, our Quality Assurance department, through the use of our corrective action program, drives and documents continuous performance improvement of our suppliers and internal departments. Our Quality Assurance department also trains internal auditors to audit our adherence to the Quality Management system. Our Quality Management system has been certified to International Standards Organization, or ISO, 13485:2012 by Intertek, a Notified Body to ISO.
As a medical device manufacturer, our manufacturing facilities are subject to periodic inspection by the FDA and certain corresponding state agencies. We have been audited three times since April 2014 by the FDA and found to be in compliance with Good Manufacturing Practices guidelines. We have completed two surveillance audits by our notifying body over the same period and identified one minor non-conformance, which was addressed through the implementation of a new training software. Additionally, we have had two unannounced inspections by state inspectors from California and Texas within the past year and were determined to be in complete compliance with state health and safety requirements.
As of December 31, 2013, we had approximately 76 employees in operations, manufacturing and quality assurance.
Research and development
We are committed to ongoing research and development to stay at the forefront of patient preference in the oxygen concentrator field. As of December 31, 2013, our research and development staff included 16 engineers and scientists with expertise in air separation, compressors, pneumatics, electronics, embedded software, mechanical design, sensors and manufacturing technologies. Our current research and development efforts are focused primarily on increasing functionality, improving design for ease-of-use, and reducing production costs of our Inogen One systems, as well as development of our next-generation oxygen concentrators. Over the last 3 fiscal years, Inogen has invested over $6.5 million to efficiently bring two new generations of portable oxygen concentrators to market ($2.4, $2.3 and $1.8 million for the years ended 2013, 2012 and 2011), leveraging our 27 issued U.S. patents and one issued Canadian patent while also reducing the product manufacturing costs by 40% from the 2009 and 2013.
Utilizing lean product development methodologies, we have released three generations of disruptive products over the last 10 years into the marketplace, including our Inogen One G1 in October 2004, our Inogen One G2 in March 2010, and our Inogen One G3 in September 2012. Our dedication to continuous improvement has also resulted in three mid-cycle product updates and numerous incremental improvements. Development projects utilize a combination of rapid prototyping and accelerated life testing methods to ensure products are taken from concept to commercialization in a fast and capital efficient manner. We leverage our direct patient expertise to rapidly gain insight from end users and to identify areas of innovation that lead to higher-quality products and lower total cost of ownership for its products.
Our product pipeline consists of both a stationary concentrator and a fourth generation, ultra-lightweight portable oxygen concentrators. The stationary concentrator, which we are calling Inogen At Home, will allow us to access the non-ambulatory patients market and will serve as a backup to our Inogen One patients. We currently provide a backup source of oxygen to our patients who are able to elect either a stationary concentrator or oxygen tank as their backup source. We are not able to bill or be reimbursed for these backup sources and we supply them at our own cost, which is included in our cost of rental revenues. These backup sources are currently acquired from third parties; however, upon the launch of our Inogen At Home product, we will be manufacturing and supplying these stationary backup sources. The Inogen At Home 510(k) submission was cleared by the FDA’s Devices and Radiological Health Document Control Center in June 2014. We expect to commercialize Inogen At Home in the fourth quarter of 2014. Our fourth-generation portable oxygen concentrators will be smaller and lighter than our Inogen One G3 and we expect to commercialize this product in the next twelve to eighteen months. Additionally, we continue to focus our efforts on other design and functionality improvements that enhance patient quality of life.
Competition
The oxygen therapy market is a highly competitive industry. We compete with a number of manufacturers and distributors of portable oxygen concentrators, as well as providers of other oxygen therapy solutions such as home delivery of oxygen tanks, or cylinders.
Our significant manufacturing competitors are Invacare Corporation, Respironics (a subsidiary of Koninklijke Philips N.V.), AirSep Corporation and SeQual Technologies (subsidiaries of Chart Industries, Inc.), Inova Labs, Inc. and DeVilbiss Healthcare. Given the relatively low barriers to entry in the oxygen therapy device manufacturing market, we expect that the industry will become increasingly competitive in the future. Manufacturing companies compete for sales to providers primarily on the basis of product features, service and price. We believe our manufacturing competitors’ complete reliance on home medical equipment distribution compresses their margins and limits their ability to invest in product features that address consumer preferences. To pursue a direct-to-
-49-
consumer strategy, our manufacturing competitors would need to meet national accreditation and state-by-state licensing requirements and secure Medicare billing privileges, as well as compete directly with the home medical equipment providers that many rely on across their entire homecare businesses. For our two largest medical device competitors, their entire oxygen business, including stationary, transfilling, and portable oxygen concentrators, represents less than 14% percent of their billion-dollar plus homecare businesses.
Lincare Inc., Apria Healthcare, Inc. Rotech Healthcare, Inc. and American HomePatient, Inc. have been among the market leaders in providing oxygen therapy for many years, while the remaining oxygen therapy market is serviced by local providers. Because many oxygen therapy providers were either excluded from contracts in the Medicare competitive bidding process, or will have difficulty providing service at the prevailing Medicare reimbursement rates, we expect more industry consolidation. Oxygen therapy providers compete primarily on the basis of product features and service, rather than price, since reimbursement levels are established by Medicare and Medicaid, or by the individual determinations of private payors. We believe that the investment made by oxygen therapy providers in the physical distribution required for oxygen delivery limits their ability to easily switch their business model and employ a solution directly competitive to Inogen.
Some of our competitors are large, well-capitalized companies with greater resources than we have. As a consequence, they are able to spend more aggressively on product development, marketing, sales and other product initiatives than we can. Some of these competitors have:
|
• |
significantly greater name recognition; |
|
• |
established relations with healthcare professionals, customers and third-party payors; |
|
• |
established distribution networks; |
|
• |
additional lines of products, and the ability to offer rebates or bundle products to offer higher discounts or other incentives to gain a competitive advantage; |
|
• |
greater history in conducting research and development, manufacturing, marketing and obtaining regulatory approval for oxygen device products; and |
|
• |
greater financial and human resources for product development, sales and marketing, patent litigation and customer financing. |
As a result, our competitors may be able to respond more quickly and effectively than we can to new or changing opportunities, technologies, standards or customer requirements. In light of these advantages that our competitors maintain, even if our technology and direct-to-consumer distribution strategy is more effective than the technology and distribution strategy of our competitors, current or potential customers might accept competitor products and services in lieu of purchasing our products. We anticipate that we will face increased competition in the future as existing companies and competitors develop new or improved products and distribution strategies and as new companies enter the market with new technologies and distribution strategies. We may not be able to compete effectively against these organizations. Our ability to compete successfully and to increase our market share is dependent upon our reputation for providing responsive, professional and high-quality products and services and achieving strong customer satisfaction. Increased competition in the future could adversely affect our revenue, revenue growth rate, if any, margins and market share.
Government regulation
Inogen One systems, Inogen At Home system and related accessories are medical devices subject to extensive and ongoing regulation by the FDA, as well as other federal and state regulatory bodies in the United States and comparable authorities in other countries. The FDA regulations govern the following activities that we perform, or that are performed on our behalf, to ensure that medical products distributed domestically or exported internationally are safe and effective for their intended uses: product design and development, pre-clinical and clinical testing, manufacturing, labeling, storage, pre-market clearance or approval, record keeping, product marketing, advertising and promotion, sales and distribution, and post-marketing surveillance.
FDA’s pre-market clearance and approval requirements
Unless an exemption applies, each medical device we seek to commercially distribute in the United States will require either a prior 510(k) clearance or a pre-market approval from the FDA. Medical devices are classified into one of three classes—Class I, Class II or Class III—depending on the degree of risk associated with each medical device and the extent of control needed to ensure safety and effectiveness. Devices deemed to pose lower risks are placed in either Class I or II, which requires the manufacturer to submit to the FDA a premarket notification requesting permission to commercially distribute the device. This process is generally known as 510(k) clearance. Some low risk devices are exempted from this requirement. Devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a previously cleared 510(k) device, are placed in Class III, requiring premarket approval.
-50-
510(k) clearance pathway
When a 510(k) clearance is required, we must submit a premarket notification to the FDA demonstrating that our proposed device is substantially equivalent to a previously cleared and legally marketed 510(k) device or a device that was in commercial distribution before May 28, 1976 for which the FDA has not yet called for the submission of a pre-market approval application. By regulation, the FDA is required to clear or deny a 510(k) premarket notification within 90 days of submission of the application. As a practical matter, clearance often takes significantly longer. The FDA may require further information, including clinical data, to make a determination regarding substantial equivalence. If the FDA determines that the device, or its intended use, is not substantially equivalent to a previously-cleared device or use, the FDA will place the device, or the particular use, into Class III. We obtained 510(k) clearance for the original Inogen One system on May 13, 2004. We market the Inogen One G2 and G3 systems pursuant to the original Inogen One 510(k) clearance. We obtained 510(k) clearance for the Inogen At Home System on June 20, 2014.
Pre-market approval pathway
A pre-market approval application must be submitted to the FDA if the device cannot be cleared through the 510(k) process. The pre-market approval application process is much more demanding than the 510(k) premarket notification process. A pre-market approval application must be supported by extensive data, including but not limited to technical, preclinical, clinical trials, manufacturing and labeling to demonstrate to the FDA’s satisfaction reasonable evidence of safety and effectiveness of the device.
After a pre-market approval application is submitted and the FDA determines that the application is sufficiently complete to permit a substantive review, the FDA will accept the application for review. The FDA has 180 days to review an “accepted” pre-market approval application, although the review of an application generally occurs over a significantly longer period of time and can take up to several years. During this review period, the FDA may request additional information or clarification of the information already provided. Also, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. In addition, the FDA will conduct a preapproval inspection of the manufacturing facility to ensure compliance with quality system regulations.
Clinical trials
Clinical trials are almost always required to support pre-market approval and are sometimes required for 510(k) clearance. In the United States, these trials generally require submission of an application for an Investigational Device Exemption, or IDE, to the FDA. The IDE application must be supported by appropriate data, such as animal and laboratory testing results, showing it is safe to test the device in humans and that the testing protocol is scientifically sound. The IDE must be approved in advance by the FDA for a specific number of patients unless the product is deemed a non-significant risk device eligible for more abbreviated IDE requirements. Clinical trials for significant risk devices may not begin until the IDE application is approved by the FDA and the appropriate institutional review boards, or IRBs, at the clinical trial sites. We, the FDA or the IRB at each site at which a clinical trial is being performed may suspend a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the benefits. Even if a trial is completed, the results of clinical testing may not demonstrate the safety and efficacy of the device, may be equivocal or may otherwise not be sufficient to obtain approval or clearance of the product.
Pervasive and ongoing regulation by the FDA
Even after a device receives clearance or approval and is placed on the market, numerous regulatory requirements apply. These include:
|
• |
establishment registration and device listing; |
|
• |
quality system regulation, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the manufacturing process; |
|
• |
labeling regulations and the FDA prohibitions against the promotion of products for un-cleared, unapproved or “off-label” uses, and other requirements related to promotional activities; |
|
• |
medical device reporting regulations, which require that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur; |
|
• |
corrections and removals reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the Federal Food, Drug and Cosmetic Act that may present a risk to health; and |
|
• |
post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device. |
-51-
After a device receives 510(k) clearance or a pre-market approval, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, will require a new clearance or approval. The FDA requires each manufacturer to make this determination initially, but the FDA can review any such decision and can disagree with a manufacturer’s determination. We have modified various aspects of our Inogen One systems since receiving regulatory clearance, but we believe that new 510(k) clearances are not required for these modifications. If the FDA disagrees with our determination not to seek a new 510(k) clearance, the FDA may retroactively require us to seek 510(k) clearance or pre-market approval. The FDA could also require us to cease marketing and distribution and/or recall the modified device until 510(k) clearance or pre-market approval is obtained. Also, in these circumstances, we may be subject to significant regulatory fines and penalties.
Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following sanctions: Warning Letters, fines, injunctions, civil or criminal penalties, recall or seizure of our products, operating restrictions, partial suspension or total shutdown of production, refusing our request for 510(k) clearance or pre-market approval of new products, rescinding previously granted 510(k) clearances or withdrawing previously granted pre-market approvals.
We are subject to announced and unannounced inspections by the FDA, and these inspections may include the manufacturing facilities of our subcontractors. Inogen has been audited three times since April 2014 by the FDA and found to be in compliance with the Quality System Regulation. We cannot assure you that we can maintain a comparable level of regulatory compliance in the future at our facility.
International sales of medical devices are subject to foreign government regulations, which may vary substantially from country to country. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA approval, and the requirements may differ. There is a trend towards harmonization of quality system standards among the European Union, United States, Canada and various other industrialized countries.
Licensure
In April 2009, we became a Durable, Medical Equipment, Prosthetics, Orthotics, and Supplies accredited Medicare supplier by Accreditation Commission for Health Care for our Goleta, California facility for Home/Durable Medical Equipment Services for oxygen equipment and supplies. Our Medicare accreditation must be renewed every three years through passage of an on-site inspection. Our current accreditation with Medicare is due to expire in May 2015. Several states require that durable medical equipment providers be licensed in order to sell products to patients in that state. Certain of these states require that durable medical equipment providers maintain an in-state location. Most of our state licenses are renewed on an annual or bi-annual basis. Although we believe we are in compliance with all applicable state regulations regarding licensure requirements, if we were found to be noncompliant, we could lose our licensure in that state, which could prohibit us from selling our current or future products to patients in that state. In addition, we are subject to certain state laws regarding professional licensure. We believe that our certified clinicians are in compliance with all such state laws. If our clinicians were to be found non-compliant in a given state, we would need to modify our approach to providing education, clinical support and customer service in such state.
Federal anti-kickback and self-referral laws
The Federal Anti-Kickback Statute prohibits the knowing and willful offer, payment, solicitation or receipt of any form of remuneration in return for, or to induce the:
|
• |
referral of a person; |
|
• |
furnishing or arranging for the furnishing of items or services reimbursable under Medicare, Medicaid or other governmental programs; or |
|
• |
purchase, lease, or order of, or the arrangement or recommendation of the purchasing, leasing, or ordering of any item or service reimbursable under Medicare, Medicaid or other governmental programs. |
The Federal Anti-Kickback Statute applies to our arrangements with sales representatives, customers and healthcare providers, as well as certain coding and billing information that we may provide to purchasers of Inogen One systems. Although we believe that we have structured such arrangements to be in compliance with the Anti-Kickback Statute and other applicable laws, regulatory authorities may determine otherwise. Noncompliance with the federal anti-kickback statute can result in exclusion from Medicare, Medicaid or other governmental programs, restrictions on our ability to operate in certain jurisdictions, as well as civil and criminal penalties, any of which could have an adverse effect on our business and results of operations.
Federal law also includes a provision commonly known as the “Stark Law,” which prohibits a physician from referring Medicare or Medicaid patients to an entity providing “designated health services,” including a company that furnishes durable medical equipment, in which the physician has an ownership or investment interest or with which the physician has entered into a compensation
-52-
arrangement. Violation of the Stark Law could result in denial of payment, disgorgement of reimbursements received under a noncompliant arrangement, civil penalties, and exclusion from Medicare, Medicaid or other governmental programs. Although we believe that we have structured our provider arrangements to comply with current Stark Law requirements, these arrangements may not expressly meet the requirements for applicable exceptions from the law.
Additionally, as some of these laws are still evolving, we lack definitive guidance as to the application of certain key aspects of these laws as they relate to our arrangements with providers with respect to patient training. We cannot predict the final form that these regulations will take or the effect that the final regulations will have on us. As a result, our provider arrangements may ultimately be found to be not in compliance with applicable federal law.
Federal false claims act
The Federal False Claims Act provides, in part, that the federal government may bring a lawsuit against any person whom it believes has knowingly presented, or caused to be presented, a false or fraudulent request for payment from the federal government, or who has made a false statement or used a false record to get a claim approved. In addition, amendments in 1986 to the Federal False Claims Act have made it easier for private parties to bring “qui tam” whistleblower lawsuits against companies. Although we believe that we are in compliance with the federal government’s laws and regulations, if we are found in violation of these laws, penalties include fines ranging from $5,500 to $11,000 for each false claim, plus three times the amount of damages that the federal government sustained because of the act of that person. We believe that we are in compliance with the federal government’s laws and regulations concerning the filing of reimbursement claims.
Civil monetary penalties law
The Federal Civil Monetary Penalties Law prohibits the offering or transferring of remuneration to a Medicare or Medicaid beneficiary that the person knows or should know is likely to influence the beneficiary’s selection of a particular supplier of Medicare or Medicaid payable items or services. We sometimes offer customers various discounts and other financial incentives in connection with the sales of our products. While it is our intent to comply with all applicable laws, the government may find that our marketing activities violate the Civil Monetary Penalties Law. If we are found to be in noncompliance, we could be subject to civil money penalties of up to $10,000 for each wrongful act, assessment of three times the amount claimed for each item or service and exclusion from the Federal healthcare programs. In addition, to the extent we are found to not be in compliance, we may be required to curtail or restructure our operations. Any penalties, damages, fines, exclusions, curtailment or restructuring of our operations could adversely affect our ability to operate our business and our financial results.
State fraud and abuse provisions
Many states have also adopted some form of anti-kickback and anti-referral laws and false claims act that may apply to all payors. We believe that we are in compliance with such laws. Nevertheless, a determination of liability under such laws could result in fines and penalties and restrictions on our ability to operate in these jurisdictions.
HIPAA
In addition to creating the two new federal healthcare crimes, regulations implementing HIPAA also establish uniform standards governing the conduct of certain electronic healthcare transactions and protecting the security and privacy of individually identifiable health information maintained or transmitted by healthcare providers, health plans and healthcare clearinghouses, which are referred to as covered entities. Three standards have been promulgated under HIPAA’s regulations: the Standards for Privacy of Individually Identifiable Health Information, which restrict the use and disclosure of certain individually identifiable health information, the Standards for Electronic Transactions, which establish standards for common healthcare transactions, such as claims information, plan eligibility, payment information and the use of electronic signatures, and the Security Standards, which require covered entities to implement and maintain certain security measures to safeguard certain electronic health information, including the adoption of administrative, physical and technical safeguards to protect such information.
In 2009, Congress passed the American Recovery and Reinvestment Act of 2009, or ARRA, which included sweeping changes to HIPAA, including an expansion of HIPAA’s privacy and security standards. ARRA includes HITECH, which, among other things, made HIPAA’s privacy and security standards directly applicable to business associates of covered entities effective February 17, 2010. A business associate is a person or entity that performs certain functions or activities on behalf of a covered entity that involve the use or disclosure of protected health information in connection with recognized healthcare operations activities. As a result, business associates are now subject to significant civil and criminal penalties for failure to comply with applicable standards. Moreover, HITECH creates a new requirement to report certain breaches of unsecured, individually identifiable health information and imposes penalties on entities that fail to do so. HITECH also increased the civil and criminal penalties that may be imposed against covered entities, business associates and possibly other persons and gave state attorneys general new authority to file civil
-53-
actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney fees and costs associated with pursuing federal civil actions. The 2013 final HITECH omnibus rule modifies the breach reporting standard in a manner that will likely make more data security incidents qualify as reportable breaches.
In addition to federal regulations issued under HIPAA, some states have enacted privacy and security statutes or regulations that, in some cases, are more stringent than those issued under HIPAA. In those cases, it may be necessary to modify our planned operations and procedures to comply with the more stringent state laws. If we fail to comply with applicable state laws and regulations, we could be subject to additional sanctions. Any liability from failure to comply with the requirements of HIPAA, HITECH or state privacy and security statutes or regulations could adversely affect our financial condition. The costs of complying with privacy and security related legal and regulatory requirements are burdensome and could have a material adverse effect on our results or operations.
International regulation
International sales of medical devices are subject to foreign governmental regulations, which vary substantially from country to country. The time required to obtain clearance or approval by a foreign country may be longer or shorter than that required for FDA clearance or approval, and the requirements may be different.
The primary regulatory environment in Europe is that of the European Union, which has adopted numerous directives and has promulgated voluntary standards regulating the design, manufacture, clinical trials, labeling and adverse event reporting for medical devices. Devices that comply with the requirements of a relevant directive will be entitled to bear the CE conformity marking, indicating that the device conforms with the essential requirements of the applicable directives and, accordingly, can be commercially distributed throughout the member states of the European Union, and other countries that comply with or mirror these directives. The method of assessing conformity varies depending on the type and class of the product, but normally involves a combination of self-assessment by the manufacturer and a third-party assessment by a notified body, an independent and neutral institution appointed by a country to conduct the conformity assessment. This third-party assessment may consist of an audit of the manufacturer’s quality system and specific testing of the manufacturer’s device. Such an assessment may be required in order for a manufacturer to commercially distribute the product throughout these countries. ISO 9001 and ISO 13845 certifications are voluntary standards. Compliance establishes the presumption of conformity with the essential requirements for a CE Marking. We have the authorization to affix the CE Mark to our products and to commercialize our devices in the European Union. Our ISO 13485 certification was issued on April 21, 2005 and our EC-Certificate was issued on March 16, 2007.
Before we can sell our devices in Canada we must submit and obtain clearance of a license application, implement and comply with ISO Standard 13485, and undergo an audit by a registrar accredited by Health Canada. On January 25, 2006, we received our Medical Device License in Canada. In Australia, we must appoint an agent sponsor who will interact on our behalf with the Therapeutics Goods Administration (TGA). We must also prepare a technical file and declaration of conformity to essential requirements under Australian law, provide evidence of CE Marking of the device and submit this information via our agent sponsor to the TGA in a Medical Device Application. On June 4, 2007, we received our Certificate for Inclusion of a Medical Device in Australia.
Intellectual property
We believe that to maintain a competitive advantage, we must develop and preserve the proprietary aspect of our technologies. We rely on a combination of patent, trademark, trade secret and other intellectual property laws, non-disclosure agreements and other measures to protect our proprietary rights. Currently, we require our employees, consultants and advisors to execute non-disclosure agreements in connection with their employment, consulting or advisory relationships with us, where appropriate. We also require our employees, consultants and advisors who we expect to work on our current or future products to agree to disclose and assign to us all inventions conceived during the work day, developed using our property or that relate to our business. Despite any measures taken to protect our intellectual property, unauthorized parties may attempt to copy aspects of our Inogen One systems or to obtain and use information that we regard as proprietary.
Patents
As of September 30, 2014 we had five pending U.S. patent applications, 27 issued patents and one issued Canadian patent relating to design and construction of our oxygen concentrators and our intelligent delivery technology. We anticipate it will take several years for the most recent of these U.S. patent applications to result in issued patents, if at all.
-54-
Our patent portfolio contains three principal sets of patents and patent applications. The first set relates to the construction and design of specific Inogen products. For example, U.S. Patent Nos. 8,440,004; 8,366,815; 8,377,181; and 8,568,519 are directed to design elements of the Inogen One G2 portable oxygen concentrator. These patents expire in 2031 (without taking into account any patent term adjustments) and may serve to deter competitors from reverse engineering or copying our design elements. This set of patents and patent applications also contains a pending U.S. patent application that relates to the design of the Inogen One G3 portable oxygen concentrator.
The second set of patents and patent applications within our portfolio pertains to operating algorithms and design optimization techniques. U.S. Patent Nos. 7,841,343; 7,585,351; 7,857,894; 8,142,544; and 6,605,136 are directed to optimization of the Pressure Swing Adsorption oxygen generating system and the oxygen conserving technology used across all of our products. These patents expire in 2027, 2026, 2027, 2026 and 2022 respectively (without taking into account any patent term adjustments). These algorithms and optimization techniques are developed to facilitate the design and manufacturing of our products. These patents may prevent competitors from achieving the same levels of optimization as found in our products.
The third set of patents and patent applications includes system component designs that may be incorporated into our products. For example, U.S. Patent No. 8,580,015, which expires in 2027 (without taking into account any patent term adjustments), is directed to product improvements that have been utilized in the Inogen One and Inogen One G2 products. Also within this class of patents are U.S. Patent Nos. 7,686,870 and 7,922,789 that are directed to designs that may be utilized in future Inogen products to improve performance over current product offerings. These patents expire in 2027 and 2023 respectively (without taking into account any patent term adjustments).
Trademarks
We have registered the trademarks Inogen; Inogen One; Inogen One G2; Oxygenation; Live Life in Moments, not Minutes; Never Run Out of Oxygen; Oxygen Therapy on Your Terms; Oxygen. Anytime, Anywhere; Reclaim Your Independence; Intelligent Delivery Technology; and the Inogen design with the United States Patent and Trademark Office. We have applied with the United States Patent and Trademark Office to register the trademark Inogen at Home. We have registered the trademark Inogen in Australia, Canada, China, Mexico, and in Europe (European Community registration). We have registered the trademark Inogen One in Australia, Canada, China, Mexico, and in Europe (European Community registration). We have registered the trademark Satellite Conserver in Canada and China. We have registered the trademark Inogen at Home in Europe (European Community registration).
Legal proceedings
On November 4, 2011, we filed a lawsuit in the United States District Court for the Central District of California against Inova Labs Inc., or Defendant, for infringement of two of our patents. The case, Inogen Inc. v. Inova Labs Inc., Case No. 8:11-cv-01692-JST-AN, or the Lawsuit, involves U.S. Patent Nos. 7,841,343, entitled “Systems and Methods For Delivering Therapeutic Gas to Patients”, or the ’343 patent, and 6,605,136 entitled “Pressure Swing Adsorption Process Operation And Optimization”, or the ’136 patent. We alleged in the Lawsuit that certain of Defendant’s oxygen concentrators infringe various claims of the ’343 and ’136 patents. The Lawsuit seeks damages, injunctive relief, costs and attorneys’ fees.
The Defendant has answered the complaint, denying infringement and asserting various sets of defenses including non-infringement, invalidity and unenforceability, patent misuse, unclean hands, laches and estoppel. The Defendant also filed counterclaims against us alleging patent invalidity, non-infringement and inequitable conduct. We denied the allegations in the Defendant’s counterclaims and filed a motion to dismiss Defendant’s inequitable conduct counterclaim.
The Defendant filed a request with the U.S. Patent and Trademark Office seeking an inter partes reexamination of the ’343 and ’136 patents. The Defendant also filed a motion to stay the Lawsuit pending outcome of the reexamination. On March 20, 2012, the Court granted the Defendant’s motion to stay the Lawsuit pending outcome of the reexamination and also granted our motion to dismiss the Defendant’s inequitable conduct counterclaim.
We are party to various legal proceedings arising in the normal course of business. We carry insurance, subject to deductibles under the specified policies, to protect against losses from certain types of legal claims. At this time, we do not anticipate that any of these proceedings will$.000,0000ny.ggregate offering price of additional shares that the underwriters have the option to purchase to cover over-allotm have a material adverse effect on our business.
Facilities and property
We lease approximately 39,000 square feet of manufacturing and office space at our corporate headquarters in Goleta, California under a lease that expires in September 2015, and approximately 31,000 square feet of manufacturing and office space in Richardson, Texas under a lease that expires in December 2019. In addition, we lease office space in Smyrna, Tennessee, Corinth, Mississippi and
-55-
Huntsville, Alabama under leases expiring in August 2015, May 2015 and June 2015, respectively, and approximately 1,200, 350 and 700 square feet. In addition, we purchased a research and development laboratory and office property in Manitowoc, Wisconsin in September of 2014 with 2,500 square feet. We believe that our existing facilities are adequate to meet our business requirements for the near-term, and that additional space will be available on commercially reasonable terms, if required.
Employees
As of December 31, 2013, we had 354 full and part-time employees, including 180 in sales, marketing, clinical and client services, 76 in operations, manufacturing and quality assurance, 82 in general administration and 16 in research and development. None of our employees is represented by a collective bargaining agreement. We believe that our employee relations are good.
Corporate and available information
We were incorporated in Delaware in November 2001. Our principal executive offices are located at 326 Bollay Drive, Goleta, California 93117. Our telephone number is (805) 562-0500. Our website is www.inogen.com. Information contained on our website is not incorporated by reference into this prospectus, and should not be considered to be part of this prospectus.
Environmental matters
Our research and development and manufacturing processes involve the controlled use of hazardous materials, including flammables, toxics, and corrosives. Our research and manufacturing operations produce hazardous chemical waste products. We seek to comply with applicable laws regarding the handling and disposal of such materials. Given the small volume of such materials used or generated at our facilities, we do not expect our compliance efforts to have a material effect on our capital expenditures, earnings, and competitive position. However, we cannot eliminate the risk of accidental contamination or discharge and any resultant injury from these materials. We do not currently maintain separate environmental liability coverage and any such contamination or discharge could result in significant cost to us in penalties, damages, and suspension of our operations.
Backlog
We have no material backlog of orders.
Geographic information
During the six months ended June 30, 2014 and the years ended December 31, 2013, 2012 and 2011, all of our long-lived assets were located within the United States. Approximately 22% of our 2013 revenue, 27% of our 2012 revenue, and 26% of our 2011 came from international markets. Please see Note 2 to our audited financial statements included in our 2013 Annual Report and Note 2 in our June 2014 Quarterly Report for additional information related to our U.S. and non-U.S. revenue.
Seasonality
We believe our sales may be impacted by seasonal factors. For example, we typically experience higher sales in the second quarter, as a result of consumers traveling and vacationing during the summer months.
-56-
Executive officers and directors
Our executive officers and directors, and their ages and positions as of September 30, 2014 are as set forth below:
|
Name |
|
Age |
|
Position |
|
Raymond Huggenberger |
|
55 |
|
President, Chief Executive Officer and Director |
|
Scott Wilkinson |
|
49 |
|
Executive Vice President, Sales and Marketing |
|
Alison Bauerlein |
|
32 |
|
Executive Vice President, Finance and Chief Financial Officer, Corporate Secretary and Corporate Treasurer |
|
Matthew Scribner |
|
47 |
|
Executive Vice President, Operations |
|
Brenton Taylor |
|
33 |
|
Executive Vice President, Engineering |
|
Byron Myers |
|
35 |
|
Vice President, Marketing |
|
Heath Lukatch, Ph.D.(2) |
|
47 |
|
Chairman of the Board |
|
Timothy Petersen(1)(2) |
|
50 |
|
Director |
|
Benjamin Anderson-Ray(1) |
|
59 |
|
Director |
|
Loren McFarland(1) |
|
55 |
|
Director |
|
Heather Rider (2) |
|
55 |
|
Director |
|
(1) |
Member of our audit committee. |
|
(2) |
Member of our compensation, nominating and governance committee. |
Executive officers
Raymond Huggenberger has served as our President, Chief Executive Officer and as a member of the board of directors of Inogen since 2008. Prior to joining our company, Mr. Huggenberger held various management positions with Sunrise Medical Inc., a global manufacturer and distributor of durable medical equipment, including: President of Marketing for Sunrise’s German subsidiary from 1994 to 1996, President of Sunrise’s German division from 1998 until 2000, President of the European Operating Group from 2000 to 2002, President and Chief Operating Officer from 2002 until 2004, and President of European Operations 2006 to 2007. Mr. Huggenberger also held various management positions with McDermott and Bull Inc., an executive search firm, from 2005 to 2006 and in the healthcare division of TA Triumph Adler AG, a document process management firm, from 1996 to 1998. Mr. Huggenberger currently serves on the board of directors of Wellfount Corporation, a pharmacy services company, and previously served on the board of IYIA Technologies, a healthcare company. Mr. Huggenberger graduated from AKAD University in Rendsburg, Germany in Economics and completed the Advanced Marketing Strategies Program at INSEAD, Fontainebleau, France. The board of directors believes that he is qualified to serve as a director of Inogen because of his deep understanding of our business, operations and strategy.
Scott Wilkinson has served as our Executive Vice President, Sales and Marketing since 2008 and in this role currently oversees Inogen’s global operations in sales, marketing, customer service, product management, medical billing, and clinical services. Previously, he served as our Director of Product Management from 2005 to 2006 and Vice President, Product Management from 2006 to 2008. From 2000 to 2005, Mr. Wilkinson worked for Invacare Corporation, a designer and manufacturer of oxygen products, as a Group Product Manager and helped launch their $100 million oxygen product line segment. From 1999 to 2000, Mr. Wilkinson served as a Product Line Director with Johnson & Johnson, a healthcare company. From 1988 to 1999, Mr. Wilkinson worked as a Research Scientist, Product Manager, and Project Leader at Kimberly Clark, a consumer products company. Mr. Wilkinson received a Bachelor’s degree in Chemical Engineering from the University of Akron and an MBA from University of Wisconsin, Oshkosh.
Alison Bauerlein is a co-founder of Inogen and has served as our Chief Financial Officer since 2009 and Executive Vice President, Finance since March 2014. Ms. Bauerlein has also served as Corporate Secretary and Corporate Treasurer since 2002. Ms. Bauerlein previously served as our Vice President, Finance from 2008 until March 2014. Prior to serving in these positions, Ms. Bauerlein also served as Controller with our company from 2001 to 2004 and 2008 to 2009, and Director of Financial Planning and Analysis from 2004 to 2008. During her time with our company, Ms. Bauerlein has helped the company raise approximately $91 million in venture capital funding. Ms. Bauerlein currently serves on the board of directors of Active Life Scientific, Inc. Ms. Bauerlein received a Bachelor of Arts degree in Economics/Mathematics with high honors from the University of California, Santa Barbara.
-57-
Matthew Scribner has served as our Executive Vice President, Operations since March 2014, and previously served as our Vice President, Operations from 2008 until March 2014. Previously, he served as our Director of Supply Chain from 2004 to 2007 and Director of Manufacturing from 2007 to 2008. From 1998 to 2004, Mr. Scribner worked for Computer Motion, a manufacturer of surgical robots that was acquired by Intuitive Surgical, in various executive capacities, including as a Manufacturing Manager and as a Project Manager. From 1989 to 2013, Mr. Scribner also served in the United States Navy as a helicopter pilot, on both active duty and as a reservist. He was mobilized and deployed to Iraq in 2003 to fly in support of Operation Iraqi Freedom. He achieved the rank of Commander and retired from the U.S. Navy in July 2013. Mr. Scribner received a Bachelor of Science degree in Ocean Engineering from the United States Naval Academy. Mr. Scribner also received an MBA from the University of San Diego.
Brenton Taylor is a co-founder of Inogen and has served as our Executive Vice President, Engineering since March 2014. He previously served as our Vice President, Engineering from 2008 until March 2014. Prior to serving in this position, Mr. Taylor served as Director of Technology with our company from 2003 to 2008. Mr. Taylor is listed as an inventor on 23 of the company’s U.S. patents related to portable oxygen concentrator development. Mr. Taylor received a Bachelor of Science degree in Microbiology from the University of California, Santa Barbara.
Byron Myers is a co-founder of Inogen and has served as our Vice President, Marketing since 2011. In this role, Mr. Myers leads Inogen’s Marketing Department and Direct to Consumer sales channel. Prior to serving in this position, Mr. Myers held various roles with our company, including: Product Manager from 2002 to 2006, Director of Marketing from 2006 to 2007 and 2008 to 2011, International Product Manager during 2007, and Director of International Product Management from 2007 to 2008. Mr. Myers received a Bachelor’s degree in Economics/Mathematics from the University of California, Santa Barbara and an MBA from the Rady School of Management at the University of California, San Diego.
Board of directors
Heath Lukatch, Ph.D. has served as chairman of our board of directors since 2008, and as a director since 2006. Dr. Lukatch is employed as a Partner at Novo Ventures (US) Inc., which provides certain consultancy services to Novo A/S. Dr. Lukatch joined Novo Ventures (US) Inc. in 2006. Prior to joining Novo Ventures (US) Inc., Dr. Lukatch was a Managing Director responsible for biotechnology venture investments at Piper Jaffray Ventures and SightLine Partners, a private equity firm and spin off of Piper Jaffray Ventures, from 2001 to 2006. Prior to joining Piper Jaffray Ventures, Dr. Lukatch worked as a strategy consultant with McKinsey & Company, a consulting firm, from 1997 to 2000. Dr. Lukatch also served as co-founder and chief executive officer of AutoMate Scientific, a biotechnology instrumentation company from 1991 to 1997, and held scientific positions with Chiron Corporation, a biotechnology company, from 1990 to 1991, Roche Bioscience, a healthcare company, from 1996 to 1997, and Cetus Corporation, a biotechnology company, in 1987. He currently serves on the boards of directors of AnaptysBio, Inc., Cianna Medical, Inc., Flexion Therapeutics, Inc., FLAPCo LLC, and Panmira Pharmaceuticals LLC. Dr. Lukatch previously served on the boards of directors of Amira Pharmaceuticals, Elevation Pharmaceuticals, Inc., FoldRx Pharmaceuticals, Inc., InSound Medical, Inc., NeuroTherapeutics Pharma, Inc., Synosia Therapeutics, Inc., and Verax Biomedical, Inc. Dr. Lukatch received his Ph.D. in Neuroscience from Stanford University where he was a DOD USAF Fellow, and his B.A. in Biochemistry from the University of California at Berkeley. The board of directors believes that he is qualified to serve as a director of Inogen because of his extensive industry experience and experience as a venture capital investor and a board member for several venture-backed healthcare companies.
Timothy Petersen has served as a member of the board of directors of Inogen since 2010. He has been a managing director at Arboretum Ventures, a venture capital firm, since 2002. Prior to joining Arboretum, he was the managing director of the Zell Lurie Institute for Entrepreneurial Studies at the University of Michigan from 1999 to 2002. During his tenure at the University of Michigan, he also directed the Wolverine Venture Fund, the Institute’s venture capital fund focusing on early-stage life science and technology investments. Prior to the University of Michigan, Mr. Petersen was a manager in the investment banking practice at Plante Moran Corporate Finance, a professional services and consulting firm, and served as a management consultant at Industrial Economics, Inc., a consulting firm. He currently serves on the boards of Advanced ICU Care, Inc., IntelliCyt Corp., Fidelis SeniorCare, Inc., Tangent Medical Technologies, Inc., My Health Direct, Inc., CerviLenz, Inc. and KFx Corporation. Previously, Mr. Petersen served on the boards of HealthMedia, Inc. (sold to Johnson & Johnson), KFx Medical Corp., PathCentral, Inc., and Accuri Cytometers, Inc (sold to Becton, Dickinson and Company). Mr. Petersen earned a BA in Economics from Williams College. He also holds an MS in Economics from the University of Wisconsin-Madison, and an MBA from the Ross School of Business at the University of Michigan. The board of directors believes that he is qualified to serve as a director of Inogen because of his extensive experience as an investor and board member for various healthcare companies.
-58-
Benjamin Anderson-Ray has served as a member of the board of directors since 2013. He has been a partner and advisor with Trinitas Advisors, a consulting firm, since 2009. Prior to joining Trinitas Advisors, he served as the chief executive officer of three manufacturing companies: Hubbardton Forge, LLC from 2008 to 2009, Chromcraft Revington, Inc. from 2005 to 2008 and Gravograph New Hermes from 2002 to 2004. Prior to that, Mr. Anderson-Ray held various senior leadership roles at Sunrise Medical, a medical equipment manufacturer, including president of the Global Business Group in 2001, president of the Continuing Care Group from 1998 to 2000, and president of the Mobility Products Division from 1996 to 2001. Earlier in his career, Mr. Anderson-Ray held management and marketing roles at GE Lighting, a lighting solutions company, from 1984 to 1993, Black & Decker Home Products, a product manufacturing company, from 1993 to 1994, and Rubbermaid Home Products, a manufacturer and distributor of household items, from 1994 to 1996. He currently serves on the boards of 5i Science, the Episcopal Church Foundation, and the Addison County Economic Development Corporation. Previously, Mr. Anderson-Ray served on the board of Briggs Plant Propagation. Mr. Anderson-Ray has Bachelor’s degrees in Marketing and Horticulture from Michigan State University, an MBA from the University of Michigan, and is a Certified Advisor with The CEO Advantage. The board of directors believes that he is qualified to serve as a director of Inogen because of his leadership experience and his extensive industry experience.
Loren McFarland has served as a member of the board of directors of Inogen since 2013. He has been president and managing member of Santa Barbara Financial Services, LLC since 2008. Prior to founding Santa Barbara Financial Services, he served as the chief financial officer and treasurer of Mentor Corporation, a medical equipment company (now Ethicon, Inc., a Johnson & Johnson company), from 2004 to 2007. Prior to that, Mr. McFarland fulfilled various finance and accounting roles at Mentor from 1985 to 2004. He worked as a certified public accountant and audit supervisor with Touche Ross, an accounting firm, from 1981 to 1985 and served in the North Dakota Army National Guard from 1978 to 1984. He currently serves on the board of Cure Medical, LLC, a privately held manufacturer of disposable urology products, and on the board and executive committee of the MIT Enterprise Forum of the Central Coast. Previously, Mr. McFarland served on the board of directors of Patient Safety Technologies, Inc. (PSTX) as the financial expert on the audit committee and as a member of the compensation committee. Mr. McFarland has a Bachelor’s degree in accounting from the University of North Dakota and an MBA from the University of California, Los Angeles. He completed an ISS Director Certification Program in October 2008 at the University of California, Los Angeles’ Anderson School. The board of directors believes that he is qualified to serve as a director of Inogen because of his leadership experience and his extensive experience in finance and accounting.
Heather Rider has served as a member of the board of directors of Inogen since 2014. From 2012 to 2013, Ms. Rider served as Vice President, Global Human Resources of Cymer, Inc., a publicly-traded supplier of light sources for semiconductor manufacturing that was acquired by ASML Holding NV in 2013. From October 2010 to September 2012, Ms. Rider served as Senior Vice President, Global Human Resources of Alphatec Holdings, Inc., a publicly-traded medical device company focused on surgical treatment of spine disorders, and from 2006 to 2010, she served as Vice President, Human Resources of Intuitive Surgical, Inc., a publicly-traded manufacturer of robotic surgical systems. From 2001 to 2005, Ms. Rider served as Senior Vice President of Global Human Resources of Sunrise Medical, Inc., a global manufacturer and distributor of durable medical equipment. From 1998 to 2001, Ms. Rider served as Vice President of Human Resources of Biosense Webster, a member of the J & J family of companies, and a medical device manufacturer of intracardiac catheters and location technology. Prior to 1998, Ms. Rider served as Head of Human Resources for City of Hope, a leading research and treatment center for cancer, diabetes and other life-threatening diseases, CAP/MPT, a medical malpractice provider for physicians in California and medical malpractice insurance for large physician groups and hospitals, and Environmental Diagnostics International, a bio-diagnostics company with focus on the detection of environmental compounds and diseases using monoclonal antibody technology. Ms. Rider holds a B.A. in Psychology from Claremont McKenna College and an M.B.A. from Pepperdine University. The board of directors believes that she is qualified to serve as a director of Inogen because of her extensive executive-level experience with healthcare and life science companies.
There are no family relationships among any of our directors and executive officers.
Board composition and risk oversight
Our board of directors is currently composed of six directors. Five of the six directors that comprise our board of directors are independent within the meaning of the independent director guidelines of the NASDAQ Global Select Market. The certificate of incorporation and bylaws currently in effect provide that the number of directors shall be at least one and will be fixed from time to time by resolution of our board of directors.
During 2013, our board of directors met four times.
Our board of directors is divided into three classes of directors. At each annual meeting of stockholders, a class of directors will be elected for a three-year term to succeed the class whose term is then expiring. The terms of the directors will expire upon the election and qualification of successor directors at the annual meeting of stockholders to be held during the years 2015 for the Class I directors, 2016 for the Class II directors and 2017 for the Class III directors.
-59-
The Class I directors will be Timothy Petersen and Heather Rider.
The Class II directors will be Loren McFarland and Benjamin Anderson-Ray.
The Class III directors will be Heath Lukatch, Ph.D. and Raymond Huggenberger.
The division of our board of directors into three classes with staggered three-year terms may delay or prevent a change of our management or a change in control.
Our board of directors has an active role, as a whole and also at the committee level, in overseeing the management of our risks. Our board of directors is responsible for general oversight of risks and regular review of information regarding our risks, including credit risks, liquidity risks and operational risks. Our compensation, nominating and corporate governance committee is responsible for overseeing the management of risks relating to our executive compensation plans and arrangements and the risks associated with the independence of our board of directors and potential conflicts of interest. Our audit committee is responsible for overseeing the management of our risks relating to accounting matters and financial reporting. While each committee is responsible for evaluating certain risks and overseeing the management of such risks, the entire board of directors is regularly informed through discussions from committee members about such risks. Our board of directors believes its administration of its risk oversight function has not affected our board of directors’ leadership structure.
Director independence
Our common stock is listed on the NASDAQ Global Select Market. Under the rules of the NASDAQ Global Select Market, independent directors must comprise a majority of a listed company’s board of directors within a specified period after the completion of our initial public offering. In addition, the rules of the NASDAQ Global Select Market require that, subject to specified exceptions, each member of a listed company’s audit and compensation, nominating and governance committee be independent. Audit committee members must also satisfy the independence criteria set forth in Rule 10A-3 under the Securities Exchange Act of 1934, as amended, or the Exchange Act). Under the rules of the NASDAQ Global Select Market, a director will only qualify as an “independent director” if, in the opinion of that company’s board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.
To be considered independent for purposes of Rule 10A-3, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of our audit committee, our board of directors, or any other board committee: (1) accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries; or (2) be an affiliated person of the listed company or any of its subsidiaries.
In October of 2014, our board of directors undertook a review of its composition, the composition of its committees and the independence of our directors and considered whether any director has a material relationship with us that could compromise his or her ability to exercise independent judgment in carrying out his or her responsibilities. Based upon information requested from and provided by each director concerning his background, employment and affiliations, including family relationships, our board of directors has determined that none of Mr. Anderson-Ray, Ms. Rider, Dr. Lukatch, Mr. McFarland, and Mr. Petersen, representing five of our six directors, has a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each of these directors is “independent” as that term is defined under the rules of the NASDAQ Global Select Market. Our board of directors also determined that Messrs. McFarland (chairman), Petersen and Anderson-Ray, who comprise our audit committee, and Dr. Lukatch (chairman), Mr. Petersen, and Ms. Rider, who comprise our compensation, nominating and governance committee, satisfy the independence standards for those committees established by applicable Securities and Exchange Commission, or SEC, rules and the listing standards of the NASDAQ Global Select Market.
In making this determination, our board of directors considered the relationships that each non-employee director has with us and all other facts and circumstances our board of directors deemed relevant in determining independence, including the beneficial ownership of our capital stock by each non-employee director.
Board committees
Our board of directors has an audit committee and a compensation, nominating and governance committee, each of which has the composition and the responsibilities described below.
-60-
Audit committee
The members of our audit committee are Messrs. McFarland, Petersen and Anderson-Ray, each of whom is a non-employee member of our board of directors. Our audit committee chairman, Mr. McFarland, is our audit committee financial expert, as that term is defined under the SEC rules implementing Section 407 of the Sarbanes-Oxley Act of 2002, and possesses financial sophistication, as defined under the listing standards of the NASDAQ Global Select Market. Our audit committee oversees our corporate accounting and financial reporting process and assists our board of directors in monitoring our financial systems. Our audit committee will also:
|
• |
approve the hiring, discharging and compensation of our independent auditors; |
|
• |
oversee the work of our independent auditors; |
|
• |
approve engagements of the independent auditors to render any audit or permissible non-audit services; |
|
• |
review the qualifications, independence and performance of the independent auditors; |
|
• |
review our financial statements and our critical accounting policies and estimates; |
|
• |
review the adequacy and effectiveness of our internal controls; and |
|
• |
review and discuss with management and the independent auditors the results of our annual audit, our annual and quarterly financial statements and our publicly filed reports. |
Our audit committee met five times during 2013.
Compensation, nominating and governance committee
The members of our compensation, nominating and governance committee are Dr. Lukatch, Mr. Petersen and Ms. Rider. Dr. Lukatch is the chairman of our compensation, nominating and governance committee. Our compensation, nominating and governance committee oversees our compensation policies, plans and benefits programs. Our compensation, nominating and governance committee will also:
|
• |
review and recommend policies relating to compensation and benefits of our officers and employees; |
|
• |
review and approve corporate goals and objectives relevant to compensation of our chief executive officer and other senior officers; |
|
• |
evaluate the performance of our officers in light of established goals and objectives; |
|
• |
recommend compensation of our officers based on its evaluations; |
|
• |
administer the issuance of stock options and other awards under our stock plans; |
|
• |
evaluate and make recommendations regarding the organization and governance of our board of directors and its committees; |
|
• |
evaluate and propose nominees for election to our board of directors; |
|
• |
assess the performance of members of our board of directors and make recommendations regarding committee and chair assignments; |
|
• |
recommend desired qualifications for board of directors membership and conduct searches for potential members of our board of directors; and |
|
• |
review and make recommendations with respect to our corporate governance guidelines. |
Our compensation, nominating and governance committee met one time during 2013.
Our board of directors may from time to time establish other committees.
Code of ethics and conduct
We adopted a written code of ethics and conduct that applies to our directors, officers and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. A current copy of the code is posted on our website at: http://investor.inogen.com/corporate-governance.cfm.
-61-
Compensation committee interlocks and insider participation
During the past fiscal year, none of the members of our compensation, nominating and governance committee were an officer or employee of our company. None of our executive officers currently serves, or in the past year has served, as a member of the board of directors or compensation committee (or other board committee performing equivalent functions) of any entity that has one or more of its executive officers serving on our board of directors or compensation, nominating and governance committee.
Limitation of liability and indemnification
Our amended and restated certificate of incorporation and amended and restated bylaws contain provisions that limit the personal liability of our directors for monetary damages to the fullest extent permitted by Delaware law. Consequently, our directors will not be personally liable to us or our stockholders for monetary damages for any breach of fiduciary duties as directors, except liability for:
|
• |
any breach of the director’s duty of loyalty to us or our stockholders; |
|
• |
any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
|
• |
unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware General Corporation Law; or |
|
• |
any transaction from which the director derived an improper personal benefit. |
Our amended and restated certificate of incorporation provides that we indemnify our directors to the fullest extent permitted by Delaware law. In addition, our amended and restated bylaws provide that we indemnify our directors and officers to the fullest extent permitted by Delaware law. Our amended and restated bylaws also provide that we shall advance expenses incurred by a director or officer in advance of the final disposition of any action or proceeding, and permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in that capacity, regardless of whether we would otherwise be permitted to indemnify him or her under the provisions of Delaware law. We have entered and expect to continue to enter into agreements to indemnify our directors, executive officers and other employees as determined by our board of directors. With certain exceptions, these agreements provide for indemnification for related expenses including, among others, attorneys’ fees, judgments, fines and settlement amounts incurred by any of these individuals in any action or proceeding. We believe that these bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors and officers. We also maintain directors’ and officers’ liability insurance.
The limitation of liability and indemnification provisions in our amended and restated certificate of incorporation, amended and restated bylaws, and our indemnification agreements may discourage stockholders from bringing a lawsuit against our directors for breach of their fiduciary duty of care. They may also reduce the likelihood of derivative litigation against our directors and officers, even though an action, if successful, might benefit us and other stockholders. Further, a stockholder’s investment may be adversely affected to the extent that we pay the costs of settlement and damage awards against directors and officers. At present, there is no pending litigation or proceeding involving any of our directors, officers or employees for which indemnification is sought, and we are not aware of any threatened litigation that may result in claims for indemnification.
Employee benefit and stock plans
2014 Equity Incentive Plan
Our board of directors has adopted a 2014 Equity Incentive Plan, or the 2014 Plan, and our stockholders have approved it. The 2014 Plan became effective immediately prior to the effectiveness of our initial public offering. Our 2014 Plan provides for the grant of incentive stock options, within the meaning of Section 422 of the Internal Revenue Code, to our employees and any parent and subsidiary corporations’ employees, and for the grant of nonstatutory stock options, restricted stock, restricted stock units, stock appreciation rights, performance units and performance shares to our employees, directors and consultants and our parent and subsidiary corporations’ employees and consultants.
Shares Authorized
As of September 30, 2014, a total of 895,346 shares of common stock have been reserved for issuance pursuant to the 2014 Plan, of which options to purchase 630,055, shares of our common stock were outstanding, and 297,174 shares remained available for issuance. In addition, the shares to be reserved for issuance under our 2014 Plan will also include shares returned to the 2012 Plan and 2002 Plan as the result of expiration or termination of awards (provided that the maximum number of shares that may be added to the 2014 Plan pursuant to such previously granted awards under the 2012 Plan and 2002 Plan is 2,328,569 shares). The number of shares available for issuance under the 2014 Plan also includes an annual increase on the first day of each fiscal year beginning in 2015, equal to the least of:
|
• |
895,346 shares; |
-62-
|
• |
4% of the outstanding shares of common stock as of the last day of our immediately preceding fiscal year; or |
|
• |
such other amount as our board of directors may determine. |
Plan administration
Our board of directors or one or more committees appointed by our board of directors has the authority to administer the 2014 Plan. The compensation, nominating and governance committee currently administers our 2014 Plan. In the case of awards intended to qualify as “performance-based compensation” within the meaning of Section 162(m) of the Internal Revenue Code, the committee will consist of two or more “outside directors” within the meaning of Section 162(m). In addition, if we determine it is desirable to qualify transactions under the 2014 Plan as exempt under Rule 16b-3 of the Exchange Act, or Rule 16b-3, such transactions will be structured to satisfy the requirements for exemption under Rule 16b-3. Subject to the provisions of our 2014 Plan, the administrator has the power to administer the plan, including but not limited to, the power to interpret the terms of the 2014 Plan and awards granted under it, to create, amend and rescind rules and regulations relating to the 2014 Plan, including rules and regulations relating to sub-plans, and to determine the terms of the awards, including the exercise price, the number of shares subject to each such award, the exercisability of the awards, and the form of consideration, if any, payable upon exercise. The administrator also has the authority to amend existing awards to reduce or increase their exercise price, to allow participants the opportunity to transfer outstanding awards to a financial institution or other person or entity selected by the administrator, and to institute an exchange program by which outstanding awards may be surrendered in exchange for awards of the same type which may have a higher or lower exercise price or different terms, awards of a different type and/or cash.
Stock options
We may grant stock options under the 2014 Plan. The exercise price of options granted under our 2014 Plan must at least be equal to 100% of the fair market value of our common stock on the date of grant. The term of an incentive stock option may not exceed seven years, except that with respect to any participant, who owns more than 10% of the voting power of all classes of our outstanding stock, the term must not exceed five years and the exercise price must equal at least 110% of the fair market value on the grant date. The administrator will determine the methods of payment of the exercise price of an option, which may include cash, shares or other property acceptable to the administrator, as well as other types of consideration permitted by applicable law. After the termination of service of an employee, director or consultant, he or she may exercise his or her option, to the extent vested as of the termination date, for the period of time stated in his or her option agreement. Generally, if termination is due to death or disability, the option will remain exercisable for 12 months. In all other cases, the option will generally remain exercisable for three months following the termination of service. However, in no event may an option be exercised later than the expiration of its term. Subject to the provisions of our 2014 Plan, the administrator determines the other terms of options.
Stock appreciation rights
We may grant stock appreciation rights under our 2014 Plan. Stock appreciation rights allow the recipient to receive the appreciation in the fair market value of our common stock between the exercise date and the date of grant. Stock appreciation rights may not have a term exceeding seven years. After the termination of service of an employee, director or consultant, he or she may exercise his or her stock appreciation right for the period of time stated in his or her option agreement. However, in no event may a stock appreciation right be exercised later than the expiration of its term. Subject to the provisions of our 2014 Plan, the administrator determines the other terms of stock appreciation rights, including when such rights become exercisable and whether to pay any increased appreciation in cash or with shares of our common stock, or a combination thereof, except that the per share exercise price for the shares to be issued pursuant to the exercise of a stock appreciation right will be no less than 100% of the fair market value per share on the date of grant.
Restricted stock
We may grant restricted stock under our 2014 Plan. Restricted stock awards are grants of shares of our common stock that vest in accordance with terms and conditions established by the administrator. The administrator will determine the number of shares of restricted stock granted to any employee, director or consultant and, subject to the provisions of our 2014 Plan, will determine the terms and conditions of such awards. The administrator may impose whatever conditions to vesting it determines to be appropriate (for example, the administrator may set restrictions based on the achievement of specific performance goals or continued service to us); provided, however, that the administrator, in its sole discretion, may accelerate the time at which any restrictions will lapse or be removed. Recipients of restricted stock awards generally will have voting and dividend rights with respect to such shares upon grant without regard to vesting, unless the administrator provides otherwise. Shares of restricted stock that do not vest are subject to our right of repurchase or forfeiture. Notwithstanding the foregoing, the administrator, in its sole discretion, may accelerate the time at which any restrictions will lapse or be removed.
-63-
Restricted stock units
We may grant restricted stock units under our 2014 Plan. Restricted stock units are bookkeeping entries representing an amount equal to the fair market value of one share of our common stock. Subject to the provisions of our 2014 Plan, the administrator determines the terms and conditions of restricted stock units, including the vesting criteria (which may include accomplishing specified performance criteria or continued service to us) and the form and timing of payment. Notwithstanding the foregoing, the administrator, in its sole discretion, may reduce or waive any vesting criteria that must be met to receive a payout.
Performance units and performance shares
We may grant performance units and performance shares under our 2014 Plan. Performance units and performance shares are awards that will result in a payment to a participant only if performance goals established by the administrator are achieved or the awards otherwise vest. The administrator will establish organizational or individual performance goals or other vesting criteria in its discretion, which, depending on the extent to which they are met, will determine the number and/or the value of performance units and performance shares to be paid out to participants. After the grant of a performance unit or performance share, the administrator, in its sole discretion, may reduce or waive any performance criteria or other vesting provisions for such performance units or performance shares. Performance units shall have an initial dollar value established by the administrator prior to the grant date. Performance shares shall have an initial value equal to the fair market value of our common stock on the grant date. The administrator, in its sole discretion, may pay earned performance units or performance shares in the form of cash, in shares or in some combination thereof.
Outside directors
Our 2014 Plan provides that all outside directors will be eligible to receive all types of awards (except for incentive stock options) under the 2014 Plan. In October 2013, we implemented a formal policy pursuant to which our non-employee directors will be eligible to receive equity awards under the 2014 Plan. Our 2014 Plan provides that in any given fiscal year, an outside director will not receive awards covering more than 200,000 shares (increasing to 250,000 shares for the initial year of service as an outside director).
Non-transferability of awards
Unless the administrator provides otherwise, our 2014 Plan generally will not allow for the transfer of awards and only the recipient of an award may exercise an award during his or her lifetime.
Certain adjustments
In the event of certain changes in our capitalization, to prevent diminution or enlargement of the benefits or potential benefits available under the 2014 Plan, the administrator will adjust the number and class of shares that may be delivered under the 2014 Plan and/or the number, class, and price of shares covered by each outstanding award, and the numerical share limits set forth in the 2014 Plan. In the event of our proposed liquidation or dissolution, the administrator will notify participants as soon as practicable and all awards will terminate immediately prior to the consummation of such proposed transaction.
Merger or change in control
Our 2014 Plan provides that in the event of a merger or change in control, as defined under the 2014 Plan, each outstanding award will be treated as the administrator determines, except that if a successor corporation or its parent or subsidiary does not assume or substitute an equivalent award for any outstanding award, then such award will fully vest, all restrictions on such award will lapse, all performance goals or other vesting criteria applicable to such award will be deemed achieved at 100% of target levels and such award will become fully exercisable, if applicable, for a specified period prior to the transaction. The award will then terminate upon the expiration of the specified period of time. If the service of an award holder is terminated on or within the 12 months following a change in control, as a result of an involuntary termination as defined in the 2014 Plan, his or her options, restricted stock units and stock appreciation rights, if any, will vest fully and become immediately exercisable, all restrictions on his or her restricted stock will lapse, and all performance goals or other vesting requirements for his or her performance shares and units will be deemed achieved at 100% of target levels, and all other terms and conditions met.
In addition, in the event of a change in control, options, stock appreciation rights, restricted stock, and restricted stock units held by our outside directors, if any, will vest fully and become immediately exercisable, all restrictions on his or her restricted stock will lapse, and all performance goals or other vesting for his or her performance shares and units will be deemed achieved at one hundred percent (100%) of target levels, and all other terms and conditions met.
Amendment, suspension or termination
The administrator will have the authority to amend, suspend or terminate the 2014 Plan provided such action does not impair the existing rights of any participant. Our 2014 Plan will automatically terminate in 2024, unless the administrator terminates it sooner.
-64-
2012 Equity Incentive Plan
Our board of directors adopted, and our stockholders approved, our 2012 Equity Incentive Plan, or the 2012 Plan, in March 2012 and the 2012 Plan was amended and restated in October 2013. Our 2012 Plan terminated in connection with our initial public offering, and, accordingly, no shares are available for issuance under this plan. The 2012 Plan will continue to govern outstanding awards granted thereunder.
Authorized shares
An aggregate of 1,219,027 shares of our common stock was reserved for issuance under the 2012 Plan. In addition, the shares reserved for issuance under our 2012 Plan also included shares returned to the 2002 Plan as the result of expiration or termination of awards (provided that the maximum number of shares that could be added to the 2012 Plan was 1,424,646 shares). The 2012 Plan provided for the grant of incentive stock options, within the meaning of Section 422 of the Internal Revenue Code, to our employees and any parent and subsidiary corporations’ employees, and for the grant of nonstatutory stock options, restricted stock, restricted stock units, and stock appreciation rights to our employees, directors and consultants. As of September 30, 2014, options to purchase 846,425 shares of our common stock remained outstanding under the 2012 Plan.
Plan administration
Our board of directors or one or more committees appointed by our board of directors administers the 2012 Plan. The compensation, nominating and governance committee currently administers our 2012 Plan. Subject to the provisions of our 2012 Plan, the administrator has the power to administer the plan, including but not limited to, the power to: (1) determine the fair market value of our common stock; (2) determine when an option may be settled in cash; (3) implement an exchange program; (4) adjust the vesting of an option; (5) construe and interpret the 2012 Plan; and (6) modify terms of grants to non-U.S. recipients in accordance with applicable laws. The administrator may also make all other determinations deemed necessary or advisable for administering the 2012 Plan.
Options
Under the 2012 Plan, the administrator had the power to grant options. The exercise price per share of options generally had to equal at least 100% of the fair market value per share of our common stock on the date of grant. The term of an option could not exceed ten years. An incentive stock option held by a participant who owns more than 10% of the total combined voting power of all classes of our stock, or any parent or subsidiary corporations, could not have had a term in excess of ten years and must have had an exercise price of at least 110% of the fair market value per share of our common stock on the date of grant.
After the termination of service, a participant may generally exercise his or her option, to the extent vested as of such date of termination, for the period of time stated in his or her option agreement. Generally, if termination is due to disability or death, the option will remain exercisable, to the extent vested as of such date of termination, for 6 months or such longer period of time as is specified in the option agreement. In all other cases, the option generally will remain exercisable for three months following termination of service. However, in no event may an option be exercised later than the expiration of its term.
Transferability of awards
Our 2012 Plan generally does not allow for the transfer of stock options and a stock option only may be exercised during the stock option recipient’s lifetime.
Certain adjustments
In the event of certain changes in our capitalization without our receipt of consideration, the number of shares of our common stock covered by each outstanding option under the 2012 Plan and the exercise price per share of each outstanding option will be appropriately adjusted. In the event of our proposed liquidation or dissolution, all outstanding awards terminate immediately prior to such event.
Change in control
Our 2012 Plan provides that in the event of a merger or change in control (as defined in the 2012 Plan), each outstanding option will be treated as the administrator determines, except that if a successor corporation or its parent or subsidiary does not assume or substitute an equivalent award for an outstanding option, then the vesting of such options will be accelerated in full, and the options will be terminated if not exercised prior to such event. If the service of an award holder is terminated on or within the 12 months following a change in control, as a result of an involuntary termination as defined in the 2014 Plan, his or her options, restricted stock units and stock appreciation rights, if any, will vest fully and become immediately exercisable, all restrictions on his or her restricted stock will lapse, and all performance goals or other vesting requirements for his or her performance shares and units will be deemed achieved at 100% of target levels, and all other terms and conditions met.
-65-
Amendment or termination
Our board of directors may amend the 2012 Plan at any time. Our 2012 Plan terminated in connection with our initial public offering and no further awards will be granted thereunder. All outstanding options will continue to be governed by their existing terms.
2002 Stock Incentive Plan, as most recently amended in February 2010
Our board of directors adopted and approved, and our stockholders approved, our 2002 Stock Incentive Plan, or the 2002 Plan, in May 2002. Our 2002 Plan was terminated in March 2012 in connection with the adoption of our 2012 Plan and, accordingly, no shares were available for issuance under this plan after that time. The 2002 Plan continues to govern outstanding stock options granted thereunder. An aggregate of 1,983,093 shares of our common stock was reserved for issuance under the 2002 Plan. The 2002 Plan provided for the grant of incentive stock options and nonqualified stock options. As of September 30, 2014, options to purchase 1,201,848 shares of our common stock remained outstanding under the 2002 Plan.
Plan administration
Our board of directors or one or more committees appointed by our board of directors administers the 2002 Plan. The compensation, nominating and governance committee currently administers our 2002 Plan. Subject to the provisions of our 2002 Plan, the administrator has the power to administer the plan. Any action, decision, interpretation, or determination made in good faith by the administrator will be final and binding on us and all 2002 Plan participants.
Options
Under the 2002 Plan, the administrator had the power to grant options. The exercise price per share of options generally had to equal at least 100% of the fair market value per share of our common stock on the date of grant. The term of an option could not exceed 10 years. An incentive stock option held by a participant who owns more than 10% of the total combined voting power of all classes of our stock, or any parent or subsidiary corporations, could not have had a term in excess of 5 years and must have had an exercise price of at least 110% of the fair market value per share of our common stock on the date of grant.
After the termination of service, a participant may generally exercise his or her option, to the extent vested as of such date of termination, for the period of time stated in his or her option agreement. Generally, if termination is due to disability or death, the option will remain exercisable, to the extent vested as of such date of termination, for at least 6 months. If the termination is for a reason other than death, disability, or cause (as defined in the 2002 Plan), the option will remain exercisable, to the extent vested as of such date of termination, for at least 30 days.
Transferability of options
Our 2012 Plan generally does not allow for the transfer of stock options and a stock option only may be exercised during the stock option recipient’s lifetime.
Certain adjustments
In the event of certain changes in our capitalization without our receipt of consideration, the number of shares of our common stock covered by each outstanding option under the 2002 Plan and the exercise price per share of each outstanding option will be appropriately adjusted.
Change in control
Our 2002 Plan provides that in the event of a change in control (as defined in the 2002 Plan), each outstanding option will accelerate automatically, effective as of immediately prior to the change in control unless the options are to be assumed by the acquiring or successor entity (or parent thereof) or new options are to be issued in exchange thereof.
Amendment or termination
Our board of directors may amend the 2002 Plan at any time, provided that such amendment generally may not affect or impair the rights of any holder of outstanding options without the option holder’s consent. As noted above, the 2002 Plan was terminated in March 2012 and no further awards will be granted thereunder. All outstanding awards will continue to be governed by their existing terms.
2014 Employee Stock Purchase Plan
Our board of directors has adopted a 2014 Employee Stock Purchase Plan, or the ESPP, and our stockholders have approved it. The ESPP became effective immediately prior to the effectiveness of this prospectus.
-66-
Authorized shares
As of September 30, 2014, a total of 148,711 shares were available for sale under the ESPP. In addition, our ESPP provides for annual increases in the number of shares available for sale under the ESPP on the first day of each fiscal year beginning in 2015, equal to the least of:
|
• |
179,069 shares; |
|
• |
1.5% of the outstanding shares of our common stock on the last day of our immediately preceding fiscal year; or |
|
• |
such other amount as may be determined by the administrator. |
Plan administration
Our board of directors or a committee appointed by our board of directors will administer the ESPP. The compensation, nominating and governance committee currently administers the ESPP. The administrator will have authority to administer the plan, including but not limited to, full and exclusive authority to interpret the terms of the ESPP, determine eligibility to participate subject to the conditions of our ESPP as described below, and to establish procedures for plan administration necessary for the administration of the ESPP, including adopting sub-plans.
Eligibility
Generally, all of our employees will be eligible to participate if they are employed by us, or any participating subsidiary, for at least 20 hours per week and more than five months in any calendar year. However, an employee may not be granted an option to purchase stock under the ESPP if such employee:
|
• |
immediately after the grant would own stock possessing 5% or more of the total combined voting power or value of all classes of our capital stock; or |
|
• |
holds rights to purchase stock under all of our employee stock purchase plans that accrue at a rate that exceeds $25,000 worth of stock for each calendar year in which the option is outstanding. |
Offering periods
Our ESPP is intended to qualify under Section 423 of the Code, and provides for six-month offering periods. The offering periods generally start on the first trading day on or after March 1 and September 1 of each year. However, the first offering period will begin on the registration date on which this prospectus forms a part and will end on the first trading day on or after September 1, 2014. The administrator may, in its discretion, modify the terms of future offering periods subject to the terms of our ESPP.
Payroll deductions
Our ESPP will permit participants to purchase common stock through payroll deductions of up to 15% of their eligible compensation, which includes a participant’s base straight time gross earnings, incentive compensation, bonuses, overtime and shift premium, but exclusive of payments for equity compensation and other similar compensation. A participant may purchase a maximum of 1,500 shares during a purchase period.
Exercise of option
Amounts deducted and accumulated by the participant are used to purchase shares of our common stock at the end of each six-month offering period. The purchase price of the shares will be 85% of the lower of the fair market value of our common stock on the first trading day of each offering period or on the exercise date. Participants may end their participation at any time during an offering period, and will be paid their accrued payroll deductions that have not yet been used to purchase shares of common stock. Participation ends automatically upon termination of employment with us.
Non-transferability of options
A participant may not transfer rights granted under the ESPP other than by will, the laws of descent and distribution, or as otherwise provided under the ESPP.
Merger or change in control
In the event of our merger or change in control, as defined under the ESPP, a successor corporation may assume or substitute for each outstanding option. If the successor corporation refuses to assume or substitute for the option, the offering period then in progress will be shortened, and a new exercise date will be set. The administrator will notify each participant that the exercise date has been changed and that the participant’s option will be exercised automatically on the new exercise date unless prior to such date the participant has withdrawn from the offering period.
-67-
Amendment or termination
Our ESPP will automatically terminate in 2034, unless we terminate it sooner. The administrator has the authority to amend, suspend or terminate our ESPP, except that, subject to certain exceptions described in the ESPP, no such action may adversely affect any outstanding rights to purchase stock under our ESPP.
Executive incentive compensation plan
Our board of directors has adopted an Executive Incentive Compensation Plan, or the Bonus Plan. The Bonus Plan allows our compensation, nominating and governance committee to provide cash incentive awards to selected employees, including our named executive officers, based upon performance goals established by our compensation, nominating and governance committee.
Under the Bonus Plan, our compensation, nominating and governance committee will determine the performance goals applicable to any award, which goals may include, without limitation: enrollments, business divestitures and acquisitions, cash flow, cash position, customer satisfaction, earnings (which may include earnings before interest and taxes, earnings before taxes and net earnings), earnings per share, adherence to budget, expenses, gross margin, growth in stockholder value relative to the moving average of the S&P 500 Index or another index, innovation, internal rate of return, net income, net profit, net sales, new product development, new product invention or innovation, number of customers, operating cash flow, operating expenses, operating income, operating margin, overhead or other expense reduction, productivity, profit, reduce cost per enrollment, return on assets, return on capital, return on equity, return on investment, return on sales, revenue, revenue growth, sales results, sales growth, stock price, time to market, total stockholder return, working capital, and individual objectives such as peer reviews or other subjective or objective criteria and individual objectives such as peer reviews or other subjective or objective criteria. Performance goals that include the Company’s financial results may be determined in accordance with U.S. generally accepted accounting principles, or GAAP, or such financial results may consist of non-GAAP financial measures and any actual results may be adjusted by our compensation, nominating and governance committee for one-time items or unbudgeted or unexpected items when determining whether the performance goals have been met. The goals may be on the basis of any factors our compensation, nominating and governance committee determines relevant, and may be adjusted on an individual, divisional, business unit or company-wide basis. The performance goals may differ from participant to participant and from award to award.
Our compensation, nominating and governance committee may, in its sole discretion and at any time, increase, reduce or eliminate a participant’s actual award, and/or increase, reduce or eliminate the amount allocated to the bonus pool for a particular performance period. The actual award may be below, at or above a participant’s target award, in our compensation, nominating and governance committee’s discretion. Our compensation, nominating and governance committee may determine the amount of any reduction on the basis of such factors as it deems relevant, and it is not be required to establish any allocation or weighting with respect to the factors it considers.
Actual awards are paid in cash only after they are earned, which usually requires continued employment through the date a bonus is paid. Payment of bonuses occurs as soon as administratively practicable after they are earned, but no later than the dates set forth in the Bonus Plan.
Our board of directors has the authority to amend, alter, suspend or terminate the Bonus Plan provided such action does not impair the existing rights of any participant with respect to any earned bonus.
401(k) plan
We maintain a tax-qualified retirement plan that provides eligible employees with an opportunity to save for retirement on a tax advantaged basis. All participants’ interests in their deferrals are 100% vested when contributed. In 2013, 2012 and 2011, we made no matching contributions into the 401(k) plan. Pre-tax contributions are allocated to each participant’s individual account and are then invested in selected investment alternatives according to the participants’ directions. The 401(k) plan is intended to qualify under Sections 401(a) and 501(a) of the Internal Revenue Code. As a tax-qualified retirement plan, contributions to the 401(k) plan and earnings on those contributions are not taxable to the employees until distributed from the 401(k) plan, and all contributions are deductible by us when made.
-68-
Certain relationships and related party transactions
The following is a summary of transactions since January 1, 2011 to which we have been a party in which the amount involved exceeded $120,000 and in which any of our executive officers, directors, promoters or beneficial holders of more than 5% of our capital stock had or will have a direct or indirect material interest, other than compensation arrangements which are described under the section of our 2013 Annual Report captioned “Management—Director compensation” and “Executive compensation.”
Related person transaction policy
We have adopted a written Related Person Transactions Policy that sets forth our policies and procedures regarding the identification, review, consideration, approval and oversight of “related person transactions”. For purposes of our policy only, a “related person transaction” is a past, present or future transaction, arrangement or relationship (or any series of similar transactions, arrangements or relationships) in which we and any “related person” are participants, the amount involved exceeds $120,000 and a related person has a direct or indirect material interest. Transactions involving compensation for services provided to us as an employee, director, consultant or similar capacity by a related person are not covered by this policy. A “related person,” as determined since the beginning of our last fiscal year, is any executive officer, director or nominee to become director, a holder of more than 5% of our common stock, including any immediate family members of such persons. Any related person transaction may only be consummated if approved or ratified by our audit committee in accordance with the policy guidelines set forth below.
Under the policy, where a transaction has been identified as a related person transaction, management must present information regarding the proposed related person transaction to our audit committee for review and approval. In considering related person transactions, our audit committee takes into account the relevant available facts and circumstances including, but not limited to whether the terms of such transaction are no less favorable than terms generally available to an unaffiliated third party under the same or similar circumstances and the extent of the related person’s interest in the transaction. In the event a director has an interest in the proposed transaction, the director must recuse himself from the deliberations and approval process.
Private placements
Series G convertible preferred stock
In March 2012, we issued 2,840,260 shares of our series G convertible preferred stock at an issuance price of $7.0416 per share for aggregate monetary consideration of approximately $20,000,000, to a total of eight accredited investors, including Novo A/S, and entities affiliated with Arboretum Ventures, each of which hold 5% or more of our capital stock and is represented on our board of directors. In connection with the closing of our initial public offering, such shares of series G convertible preferred stock converted to common stock at a ratio of one to one. The following table summarizes purchases of series G convertible preferred stock by such investors:
|
Name of stockholder |
|
Inogen director |
|
Number of series G |
Approximate |
|
|
Novo A/S(1) |
|
Heath Lukatch, Ph.D. |
|
2,376,947 |
$ |
16,738,000 |
|
Funds affiliated with Arboretum Ventures(2)(3) |
|
Timothy Petersen |
|
426,039 |
$ |
3,000,000 |
|
(1) |
Consists of 2,376,947 shares of series G convertible preferred stock issued to Novo A/S in March 2012, at a price of $7.0416 per share in exchange for an aggregate cash purchase price of approximately $16,738,000. |
|
(2) |
Arboretum Ventures affiliates holding our securities whose shares are aggregated for purposes of reporting share ownership information in this table include Arboretum Ventures II, L.P., and Arboretum Ventures IIa, L.P. |
|
(3) |
Consists of 426,039 shares of series G convertible preferred stock issued to Arboretum Ventures affiliates in March 2012, at a price of $7.0416 per share in exchange for an aggregate cash purchase price of approximately $3,000,000. |
Investors’ rights agreement
We entered into an amended and restated investors’ rights agreement with the holders of our preferred stock, including Novo A/S, entities affiliated with Arboretum Ventures, entities affiliated with Versant Ventures, Avalon Ventures VII, L.P and AMV Partners I, L.P, which each hold 5% or more of our capital stock and of which certain of our directors or former directors are or were affiliates, and entities affiliated with Stephen E. Cooper, a former member of our board of directors. Such agreement provides, among other things, that the holders of our preferred stock are entitled to rights with respect to the registration of their shares. For a description of these registration rights, see the section of this prospectus captioned “Description of capital stock—Registration rights.”
-69-
Voting agreement
Prior to our initial public offering, the election of the members of our board of directors was governed by a voting agreement with certain of the holders of our outstanding common stock, convertible preferred stock and warrants to purchase our capital stock, including Novo A/S, entities affiliated with Arboretum Ventures, entities affiliated with Versant Ventures, Avalon Ventures VII, L.P, AMV Partners I, L.P., entities affiliated with Stephen E. Cooper, a former member of our board of directors, and Alison Bauerlein, our Executive Vice President, Finance and Chief Financial Officer. The parties to the voting agreement agreed, subject to certain conditions, to vote their shares so as to elect as directors (1) one nominee designated by Stephen E. Cooper, (2) one nominee designated by Versant Venture Capital II, L.P; (3) one nominee designated by the AMV Partners I, L.P.; (4) one nominee designated by Novo A/S and its affiliates, and (5) one nominee designated by the Arboretum Ventures 1, LLC and its affiliates. For so long as Mr. Huggenberger was employed as our chief executive officer, the parties to the voting agreement also agreed to vote their shares so as to elect Mr. Huggenberger to our board of directors. In addition, the parties to the voting agreement agreed to vote their shares to elect two individuals who are designated by a majority of the other members of the board of directors. Upon the completion of our initial public offering, the voting agreement terminated, and there are no further contractual obligations regarding the election of our directors. Our current directors will continue to serve as directors until their resignations or removal or until their successors are duly elected by the holders of our common stock.
Other transactions
We have entered into separate indemnification agreements with each of our directors and certain of our officers. For a description of these agreements, see the section of this prospectus captioned “Management—Limitation of liability and indemnification.”
We have entered into employment agreements with certain of our executive officers that, among other things, provide for certain severance and change of control benefits. For a description of employment agreements with our named executive officers, see the section of our 2013 Annual Report captioned “Executive compensation—Executive employment agreements.”
We have granted stock options to our named executive officers, other executive officers and certain of our directors. See the section of our 2013 Annual Report captioned “Executive compensation—Executive employment agreements.”
-70-
Principal and selling stockholders
The following table sets forth certain information with respect to the beneficial ownership of our common stock at September 30, 2014, as adjusted to reflect the sale of common stock offered by the selling stockholders in this offering, for:
|
• |
each person who we know beneficially owns more than 5% of our common stock; |
|
• |
each of our directors; |
|
• |
each of our named executive officers; |
|
• |
all of our directors and executive officers as a group; and |
|
• |
each selling stockholder. |
The percentage of beneficial ownership prior to the offering shown in the table is based upon 18,436,802 shares outstanding as of September 30, 2014. Beneficial ownership following the offering assumes no exercise of the underwriters’ option to purchase additional shares.
Information with respect to beneficial ownership has been furnished by each director, officer or beneficial owner of more than 5% of our common stock. We have determined beneficial ownership in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities. In addition, the rules take into account shares of common stock issuable pursuant to the exercise of stock options or warrants that are either immediately exercisable or exercisable within 60 days of September 30, 2014. These shares are deemed to be outstanding and beneficially owned by the person holding those options or warrants for the purpose of computing the percentage ownership of that person, but they are not treated as outstanding for the purpose of computing the percentage ownership of any other person. Unless otherwise indicated, the persons or entities identified in this table have sole voting and investment power with respect to all shares shown as beneficially owned by them, subject to applicable community property laws.
Except as otherwise noted below, the address for each person or entity listed in the table is c/o Inogen, Inc., 326 Bollay Drive, Goleta, California 93117.
|
|
|
Beneficial ownership |
|
|
Shares to be |
|
Beneficial shares after |
|||||||
|
Name of beneficial owner |
|
Shares |
|
|
% |
|
|
Shares |
|
Shares |
|
% |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5% Stockholders |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Novo A/S (1) |
|
|
5,549,321 |
|
|
|
27.78 |
% |
|
|
|
|
|
|
|
Entities affiliated with Versant Ventures (2) |
|
|
3,400,747 |
|
|
|
17.03 |
% |
|
|
|
|
|
|
|
Entities affiliated with Arboretum Ventures (3) |
|
|
1,915,583 |
|
|
|
9.59 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Directors and named executive officers: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Raymond Huggenberger (4) |
|
|
562,040 |
|
|
|
2.81 |
% |
|
|
|
|
|
|
|
Scott Wilkinson (5) |
|
|
174,065 |
|
|
* |
|
|
|
|
|
|
|
|
|
Alison Bauerlein (6) |
|
|
209,986 |
|
|
|
1.05 |
% |
|
|
|
|
|
|
|
Heath Lukatch |
|
|
— |
|
|
* |
|
|
|
|
|
|
|
|
|
Tim Petersen (7) |
|
|
1,919,471 |
|
|
|
9.61 |
% |
|
|
|
|
|
|
|
Benjamin Anderson-Ray (8) |
|
|
5,554 |
|
|
* |
|
|
|
|
|
|
|
|
|
Loren McFarland (9) |
|
|
9,096 |
|
|
* |
|
|
|
|
|
|
|
|
|
Heather Rider (10) |
|
|
277 |
|
|
* |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
All directors and executive officers as a group (11 Persons) (11) |
|
|
3,450,576 |
|
|
|
17.28 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(*) Less than one percent |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-71-
|
(1) |
Based on information set forth in a Form 4 filed with the SEC by Novo A/S on February 24, 2014, these shares consist of 5,419,449 shares held and 129,871 shares that may be acquired pursuant to the exercise of warrants held by Novo A/S. Novo A/S is a Danish limited liability company. The board of directors of Novo A/S, which consists of Sten Scheibye, Göran Ando, Jeppe Christiansen, Steen Riisgaard and Per Wold-Olsen, has shared investment and voting control with respect to the shares held by Novo A/S and may exercise such control only with the support of a majority of the members of the Novo A/S board of directors. As such, no individual member of the Novo A/S board of directors is deemed to hold any beneficial ownership or reportable pecuniary interest in the shares held by Novo A/S. Dr. Lukatch, a member of our board of directors, is employed as a Partner with Novo Ventures (US) Inc. Dr. Lukatch is not deemed a beneficial owner of, and does not have a reportable pecuniary interest in, the shares held by Novo A/S. The address of Novo A/S is Tuborg Havnevej 19, 2900 Hellerup, Denmark. See “Certain relationships and related party transactions” above, for additional information regarding participation in a private placement transaction in 2012. |
|
(2) |
Based on information set forth in a Form 4 filed with the SEC by individuals and entities affiliated with Versant on September 12, 2014, these shares consist of (i) 62,733 shares held by Versant Affiliates Fund II-A, L.P., a Delaware limited partnership (“VAF II-A”), (ii) 29,537 shares held by Versant Side Fund II, L.P., a Delaware limited partnership (“VSF II”), and (iii) 3,306,680 shares held by Versant Venture Capital II, L.P., a Delaware limited partnership (“VVC II”). Versant Ventures II, LLC, a Delaware limited liability company (“VV II”) serves as the sole general partner of VAF II-A, VSF II and VVC II owns no shares directly. Brian G. Atwood, Samuel D. Colella, Ross A. Jaffe, William J. Link, Ph.D., Donald B. Milder, Rebecca B. Robertson, Bradley J. Bolzon, Charles M. Warden, and Barbara N. Lubash are directors and/or members of VV II and indirectly own 1,797 shares and have voting and dispositive power over the shares held by VAF II-A, VSF II and VVC II; however, they disclaim beneficial ownership of the shares held by VAF II-A, VSF II and VVC II except to the extent of their pecuniary interests therein. The address for such entities and persons is c/o Versant Ventures, 3000 Sand Hill Road, Building 4, Suite 210, Menlo Park, California 94025. |
|
(3) |
Based on information set forth in a Form 4 filed with the SEC by individuals and entities affiliated with Arboretum on September 30, 2014, these shares consist of (i) 1,195,990 shares of common stock held of record by Arboretum Ventures II, L.P., (ii) 280,268 shares of common stock held of record by Arboretum Ventures IIa, L.P., (iii) 263,598 shares of common stock held of record by Arboretum Ventures 1, LLC, and (iv) 175,727 shares of common stock held of record by Arboretum Ventures 1-A, LLC. Arboretum Investment Manager II, LLC (“AIM II”) serves as the general partner of Arboretum Ventures II, L.P. and serves as the sole manager of Arboretum Investment Manager IIa, LLC, which serves as the general partner of Arboretum Ventures IIa, L.P. Jan Garfinkle and Timothy Petersen are the managing members of AIM II and share the power to vote or dispose of these shares and therefore each of the foregoing managing members may be deemed to have voting and investment power with respect to such shares. Arboretum Investment Manager, LLC (“AIM”) serves as the managing member of Arboretum Ventures 1, LLC and Arboretum Ventures 1-A, LLC. Jan Garfinkle and Timothy Petersen are the managing members of AIM and share the power to vote or dispose of these shares and therefore each of the foregoing managing members may be deemed to have voting and investment power with respect to such shares. The address for such entities and persons is c/o Arboretum Ventures, 303 Detroit Street, Suite 301, Ann Arbor, Michigan 48104. Timothy Petersen is a member of our board of directors. |
|
(4) |
Includes 6,808 shares held and options to purchase 555,232 shares of common stock that are exercisable within 60 days of September 30, 2014. |
|
(5) |
Includes options to purchase 174,065 shares of common stock that are exercisable within 60 days of September 30, 2014. |
|
(6) |
Includes 40,017 shares held and options to purchase 169,969 shares of common stock that are exercisable within 60 days of September 30, 2014. |
|
(7) |
Consists of the shares described in Note (3) above, and options to purchase 3,888 shares of common stock that are exercisable within 60 days of September 30, 2014. |
|
(8) |
Includes options to purchase 5,554 shares of common stock that are exercisable within 60 days of September 30, 2014. |
|
(9) |
Includes 3,125 shares held and options to purchase 5,971 shares of common stock that are exercisable within 60 days of September 30, 2014. |
|
(10) |
Includes options to purchase 277 shares of common stock that are exercisable within 60 days of September 30, 2014. |
|
(11) |
Includes 2,043,548 shares held and options to purchase 1,407,028 shares of common stock that are exercisable within 60 days of September 30, 2014. |
-72-
General
Our authorized capital stock consists of 200,000,000 shares of common stock, $0.001 par value per share, and 10,000,000 shares of preferred stock, $0.001 par value per share. The following is a description of the material terms of, and is qualified in its entirety by, our amended and restated certificate of incorporation and amended and restated bylaws, each as in effect as of the date of this prospectus, included as exhibits to the registration statement of which this prospectus forms a part, and to the provisions of applicable Delaware law.
Common stock
The holders of our common stock are entitled to one vote per share on all matters to be voted on by our stockholders. Subject to preferences that may be applicable to any outstanding shares of preferred stock, holders of common stock are entitled to receive ratably such dividends as may be declared by our board of directors out of funds legally available for that purpose. In the event of our liquidation, dissolution or winding up, the holders of common stock are entitled to share ratably in all assets remaining after the payment of liabilities, subject to the prior distribution rights of preferred stock then outstanding. Holders of common stock have no preemptive, conversion or subscription rights. There are no redemption or sinking fund provisions applicable to the common stock.
As of September 30, 2014, we had 18,436,802 shares of common stock outstanding and 34 holders of record of common stock which does not reflect holders who beneficially own our common stock held in nominee or street name or stockholders whose shares may be held in trust by other entities. In addition, as of September 30, 2014, we had outstanding stock options to purchase an aggregate of 2,678,328 shares of our common stock, at a weighted average price of $5.50 per share, outstanding under our 2002 Stock Incentive Plan, 2012 Equity Incentive Plan, and 2014 Equity Incentive Plan.
Preferred stock
Though we currently have no plans to issue any shares of preferred stock, our board of directors has the authority, without further action by our stockholders, to designate and issue up to 10,000,000 shares of preferred stock in one or more series. Our board of directors may also designate the rights, preferences and privileges of each such series of preferred stock, any or all of which may be greater than or senior to those of the common stock. Though the actual effect of any such issuance on the rights of the holders of common stock will not be known until our board of directors determines the specific rights of the holders of preferred stock, the potential effects of such an issuance include:
|
• |
diluting the voting power of the holders of common stock; |
|
• |
reducing the likelihood that holders of common stock will receive dividend payments; |
|
• |
reducing the likelihood that holders of common stock will receive payments in the event of our liquidation, dissolution, or winding up; and |
|
• |
delaying, deterring or preventing a change-in-control or other corporate takeover. |
Dividends
Subject to preferences that may be applicable to any then outstanding preferred stock, holders of common stock are entitled to receive dividends, if any, as may be declared from time to time by our board of directors out of legally available funds. For more information, see the section of this prospectus captioned “Dividend policy.”
Liquidation
In the event of our liquidation, dissolution or winding up, holders of common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities and the satisfaction of any liquidation preference granted to the holders of any then outstanding shares of preferred stock.
Rights and preferences
Holders of common stock have no preemptive, conversion, subscription or other rights, and there are no redemption or sinking fund provisions applicable to the common stock. The rights, preferences and privileges of the holders of common stock are subject to and may be adversely affected by, the rights of the holders of shares of any series of preferred stock that we may designate in the future.
-73-
Fully paid and nonassessable
All of our outstanding shares of common stock are, and the shares of common stock to be sold pursuant to this offering, when paid for, will be fully paid and nonassessable.
Warrants
As of September 30, 2014, we had the following warrants outstanding:
|
• |
warrants exercisable for an aggregate of 145,089 shares of our common stock at an exercise price of $0.30 per share issued in connection with our 2007 convertible note financing and 2009 series E convertible preferred stock financing. These warrants have various expiration dates through February 26, 2019, but expire earlier upon a change in control of our company. |
These warrants have a net exercise provision under which their holders may, in lieu of payment of the exercise price in cash, surrender the warrant and receive a net amount of shares based on the fair market value of our stock at the time of exercise of the warrants after deduction of the aggregate exercise price. These warrants contain provisions for adjustment of the exercise price and number of shares issuable upon the exercise of warrants in the event of certain stock dividends, stock splits, reorganizations, reclassifications and consolidations.
Registration rights
Under our investors’ rights agreement, following the closing of this offering, the holders of approximately shares of common stock (including the shares underlying the warrants described in “Shares Eligible for Future Sale—Warrants”) or their transferees, have the right to require us to register the offer and sale of their shares, or to include their shares in any registration statement we file, in each case as described below.
Demand registration rights
The holders of at least 50% of the shares having registration rights have the right to demand that we use best efforts to file a registration statement for the registration of the offer and sale of shares having registration rights that are requested to be registered. We are only obligated to file up to two registration statements in connection with the exercise of demand registration rights. These registration rights are subject to specified conditions and limitations, including the right of the underwriters to limit the number of shares included in any such registration under certain circumstances and our ability to defer the filing of a registration statement with respect to an exercise of such demand registration rights for up to 90 days under certain circumstances.
Form S-3 registration rights
At any time after we are qualified to file a registration statement on Form S-3, a stockholder with registration rights will have the right to demand that we file a registration statement on Form S-3 so long as the aggregate amount of shares to be offered and sold under such registration statement on Form S-3 is at least $1.0 million (net of any underwriters’ discounts or commissions). We are only obligated to file up to two registration statements on Form S-3 within a 12 month period. These registration rights are subject to specified conditions and limitations, including our ability to defer the filing of a registration statement with respect to an exercise of such Form S-3 registration rights for up to 90 days under certain circumstances.
Piggyback registration rights
If we propose to register the offer and sale of any of our securities under the Securities Act either for our own account or for the account of other stockholders, a stockholder with registration rights will have the right, subject to certain exceptions, to include their shares of common stock in the registration statement. These registration rights are subject to specified conditions and limitations, including the right of the underwriters to limit the number of shares included in any such registration statement under certain circumstances, but not below 25% of the total number of shares covered by the registration statement.
Expenses of registration
We will pay all expenses relating to any demand registrations, Form S-3 registrations and piggyback registrations, other than underwriting discounts and selling commissions.
-74-
Termination
The registration rights terminate upon the earliest of (1) the date that is five years after the closing of our initial public offering, and (2) as to a given holder of registration rights, when such holder of registration rights can sell all of such holder’s registrable securities in a 90-day period pursuant to Rule 144 promulgated under the Securities Act.
Voting rights
Under the provisions of our amended and restated certificate of incorporation, holders of our common stock are entitled to one vote for each share of common stock held by such holder on any matter submitted to a vote at a meeting of stockholders. In addition, our amended and restated certificate of incorporation provides that certain corporate actions require the approval of our stockholders. These actions, and the vote required, are as follows:
|
• |
the removal of a director requires the vote of a majority of the voting power of our issued and outstanding capital stock entitled to vote in the election of directors; and |
|
• |
the amendment of provisions of our amended and restated certificate of incorporation relating to blank check preferred stock, the classification of our directors, the removal of directors, the filling of vacancies on our board of directors, cumulative voting, and annual and special meetings of our stockholders require the vote of 66 2/3% of our then outstanding voting securities. |
Anti-takeover effects of Delaware law and our amended and restated certificate of incorporation and amended and restated bylaws
Delaware law
Certain provisions of Delaware law and our restated certificate of incorporation and bylaws contain provisions that could have the effect of delaying, deferring or discouraging another party from acquiring control of us. These provisions, which are summarized below, are expected to discourage certain types of coercive takeover practices and inadequate takeover bids. These provisions are also designed in part to encourage anyone seeking to acquire control of us to negotiate with our board of directors. We believe that the advantages gained by protecting our ability to negotiate with any unsolicited and potentially unfriendly acquirer outweigh the disadvantages of discouraging such proposals, including those priced above the then-current market value of our common stock, because, among other reasons, the negotiation of such proposals could improve their terms.
Amended and restated certificate of incorporation and amended and restated bylaws
Our amended and restated certificate of incorporation and amended and restated bylaws include provisions that:
|
• |
authorize our board of directors to issue, without further action by our stockholders, up to 10,000,000 shares of undesignated preferred stock; |
|
• |
require that any action to be taken by our stockholders be effected at a duly called annual or special meeting and not by written consent; |
|
• |
specify that special meetings of our stockholders can be called only by our board of directors, the chairman of our board of directors, the chief executive officer or the president; |
|
• |
establish an advance notice procedure for stockholder approvals to be brought before an annual meeting of our stockholders, including proposed nominations of persons for election to our board of directors; |
|
• |
provide that directors may be removed only for cause; |
|
• |
provide that vacancies on our board of directors may be filled only by a majority of directors then in office, even though less than a quorum; |
|
• |
establish that our board of directors is divided into three classes, Class I, Class II and Class III, with each class serving staggered terms; |
|
• |
specify that no stockholder is permitted to cumulate votes at any election of our board of directors; and |
|
• |
require a super majority of the stockholders and a majority of the board to amend certain of the above-mentioned provisions. |
Exclusive jurisdiction
Under the provisions of our amended and restated certificate of incorporation, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will be the sole and exclusive forum for: (i) any derivative action or proceeding brought on behalf of us; (ii) any action asserting a claim of breach of a fiduciary duty owed by any of our directors,
-75-
officers or other employees or agents to us or our stockholders; (iii) any action asserting a claim against us arising pursuant to any provision of the Delaware General Corporation Law or our amended and restated certificate of incorporation or amended and restated bylaws; or (iv) any action asserting a claim against us governed by the internal affairs doctrine. The enforceability of similar choice of forum provisions in other companies’ certificates of incorporation has been challenged in legal proceedings, and it is possible that, in connection with any action, a court could find the choice of forum provisions contained in our amended and restated certificate of incorporation to be inapplicable or unenforceable in such action.
Delaware anti-takeover statute
We are subject to the provisions of Section 203 of the Delaware General Corporation Law regulating corporate takeovers. In general, Section 203 prohibits a publicly-held Delaware corporation from engaging, under certain circumstances, in a business combination with an interested stockholder for a period of three years following the date the person became an interested stockholder unless:
|
• |
prior to the date of the transaction, our board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; |
|
• |
upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding, but not for determining the outstanding voting stock owned by the interested stockholder, (1) shares owned by persons who are directors and also officers, and (2) shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
|
• |
at/or subsequent to the date of the transaction, the business combination is approved by our board of directors of the corporation and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock which is not owned by the interested stockholder. |
Generally, a business combination includes a merger, asset or stock sale, or other transaction resulting in a financial benefit to the interested stockholder. An interested stockholder is a person who, together with affiliates and associates, owns or, within three years prior to the determination of interested stockholder status, did own 15% or more of a corporation’s outstanding voting stock. We expect the existence of this provision to have an anti-takeover effect with respect to transactions our board of directors does not approve in advance. We also anticipate that Section 203 may discourage business combinations or other attempts that might result in the payment of a premium over the market price for the shares of common stock held by our stockholders.
The provisions of Delaware law and our restated certificate of incorporation and amended and restated bylaws could have the effect of discouraging others from attempting hostile takeovers and, as a consequence, they may also inhibit temporary fluctuations in the market price of our common stock that often result from actual or rumored takeover attempts. These provisions may also have the effect of preventing changes in our management. It is possible that these provisions could make it more difficult to accomplish transactions that stockholders may otherwise deem to be in their best interests.
Transfer agent and registrar
The transfer agent and registrar for our common stock is Computershare. The transfer agent and registrar’s address is P.O. Box 43006, Providence, RI 02940-3006. The transfer agent’s telephone number is (888) 667-7671.
Listing
Our common stock has been listed on the NASDAQ Global Select Market under the symbol “INGN.”
-76-
Shares eligible for future sale
Prior to our initial public offering, there had been no public market for our common stock. Future sales of substantial amounts of common stock in the public market, including shares issued upon exercise of outstanding options, or the perception that such sales may occur, could adversely affect the market price of our common stock and also could adversely affect our future ability to raise capital through the sale of our common stock or other equity-related securities at times and prices we believe appropriate.
Upon the completion of this offering, a total of shares of common stock will be outstanding. Of these shares, all 4,511,313 shares of common stock sold in our initial public offering, all shares of common stock sold in this offering by the selling stockholders, any shares sold upon the exercise of the underwriters’ option to purchase additional shares, and any other shares that have been sold pursuant to Rule 144 under the Securities Act will be freely tradable in the public market without restriction or further registration under the Securities Act, unless these shares are held by “affiliates,” as that term is defined in Rule 144 under the Securities Act.
The remaining shares of common stock will be “restricted securities,” as that term is defined in Rule 144 under the Securities Act. These restricted securities are eligible for public sale only if they are registered under the Securities Act or if they qualify for an exemption from registration under Rules 144 or 701 under the Securities Act, which are summarized below. In addition, certain of such shares are subject to new lock-up agreements with the underwriters of this offering.
In addition, as of September 30, 2014, there were options to acquire 2,678,328 shares of our common stock under our equity plans. All of the shares of common stock expected to be sold in this offering will be freely tradable without restriction or further registration under the Securities Act unless held by our “affiliates,” as that term is defined in Rule 144 under the Securities Act.
We may issue shares of our common stock from time to time for a variety of corporate purposes, including in capital-raising activities through future public offerings or private placements, in connection with exercise of stock options, vesting of restricted stock units and other issuances relating to our employee benefit plans and as consideration for future acquisitions, investments or other purposes. The number of shares of our common stock that we may issue may be significant, depending on the events surrounding such issuances. In some cases, the shares we issue may be freely tradable without restriction or further registration under the Securities Act; in other cases, we may grant registration rights covering the shares issued in connection with these issuances, in which case the holders of the common stock will have the right, under certain circumstances, to cause us to register any resale of such shares to the public.
Lock-up agreements
In connection with this offering, we, our directors and executive officers and the selling stockholders have agreed with the underwriters that for a period of 90 days following the date of this prospectus, we and they will not, directly or indirectly, offer, sell, contract to sell, pledge, grant any option to purchase, make any short sale, or otherwise dispose of or hedge any of our shares of common stock, any options or warrants to purchase shares of our common stock, or any securities convertible into, or exchangeable for or that represent the right to receive shares of our common stock. J.P. Morgan Securities LLC, on behalf of the underwriters, may, in their sole discretion, at any time, release all or any portion of the shares from the restrictions in such agreement. These agreements are described in the section of this prospectus captioned “Underwriting.”
J.P. Morgan Securities LLC has advised us that they have no present intent or arrangement to release any shares subject to a lock-up, and will consider the release of any lock-up on a case-by-case basis. Upon a request to release any shares subject to a lock-up, J.P. Morgan Securities LLC would consider the particular circumstances surrounding the request, including, but not limited to, the length of time before the lock-up expires, the number of shares requested to be released, reasons for the request, the possible impact on the market of our common stock and whether the holder of our shares requesting the release is an officer, director or other affiliate of ours.
Rule 144
In general, under Rule 144, a person who is not our affiliate and has not been our affiliate for purposes of the Securities Act at any time during the preceding three months will be entitled to sell any shares of our common stock that such person has beneficially owned for at least six months, including the holding period of any prior owner other than one of our affiliates, subject only to the availability of current public information about us. Sales of our common stock by any such person would be subject to the availability of current public information about us if the shares to be sold were beneficially owned by such person for less than one year.
-77-
In addition, under Rule 144, a person may sell shares of our common stock acquired from us immediately upon the completion of this offering, without regard to the registration requirements of the Securities Act or the availability of public information about us, if:
|
• |
the person is not our affiliate and has not been our affiliate at any time during the preceding three months; and |
|
• |
the person has beneficially owned the shares to be sold for at least one year, including the holding period of any prior owner other than one of our affiliates. |
Our affiliates who have beneficially owned shares of our common stock for at least six months, including the holding period of any prior owner other than one of our affiliates, would be entitled to sell within any three-month period a number of shares that does not exceed the greater of:
|
• |
1% of the number of shares of our common stock then outstanding, which will equal approximately 184,000 shares immediately after this offering; and |
|
• |
the average weekly trading volume in our common stock on the NASDAQ Global Select Market during the four calendar weeks preceding the date of filing of a notice on Form 144 with respect to the sale. |
Sales under Rule 144 by one of our affiliates are also subject to manner of sale provisions and notice requirements and to the availability of current public information about us. To the extent that shares were acquired from one of our affiliates, a person’s holding period for the purpose of effecting a sale under Rule 144 would commence on the date of transfer from the affiliate.
Rule 701
Rule 701 generally allows a stockholder who purchased shares of our common stock pursuant to a written compensatory plan or contract and who is not deemed to have been one of our affiliates during the immediately preceding 90 days to sell these shares in reliance upon Rule 144, but without being required to comply with the public information, holding period, volume limitation, or notice provisions of Rule 144. Rule 701 also permits our affiliates to sell their Rule 701 shares under Rule 144 without complying with the holding period requirements of Rule 144.
Stock options
As of September 30, 2014, options to purchase an aggregate 2,678,328 shares of our common stock were outstanding. On February 18, 2014, we filed a registration statement on Form S-8 under the Securities Act to register the offer and sale of all shares of our common stock subject to outstanding stock options and all shares issuable under our stock plans. The registration statement on Form S-8 became effective immediately upon filing, and shares covered by that registration statement are eligible for sale in the public markets, subject to vesting restrictions, the lock-up agreements described above, and Rule 144 limitations applicable to affiliates.
Warrants
As of September 30, 2014, warrants to purchase an aggregate of 145,089 shares of our common stock at a weighted average exercise price of $0.30 per share, were outstanding. See “Description of capital stock—Warrants” for additional information. Such shares issued upon exercise of the warrants may be able to be sold subject to the requirements of Rule 144 described above.
Registration rights
Upon completion of this offering, the holders of approximately shares of our common stock (including the shares underlying the warrants described in “Description of capital stock—Warrants” above), will be eligible to exercise certain rights to cause us to register their shares for resale under the Securities Act, subject to various conditions and limitations. These registration rights are described under the caption “Description of capital stock—Registration Rights.” Upon the effectiveness of a registration statement covering these shares, the shares would become freely tradable, and a large number of shares may be sold into the public market. If that occurs, the market price of our common stock could be adversely affected.
-78-
Material U.S. federal income tax consequences
to non-U.S. holders of common stock
The following is a summary of the material U.S. federal income tax consequences to non-U.S. holders (as defined below) of the ownership and disposition of our common stock, but does not purport to be a complete analysis of all the potential tax considerations relating thereto. This summary is based upon the provisions of the Internal Revenue Code of 1986, as amended, or the Code, Treasury regulations promulgated thereunder, administrative rulings and judicial decisions, all as of the date hereof, all of which are subject to change, possibly with retroactive effect, which could result in U.S. federal income consequences different than those summarized below. We have not sought a ruling from the Internal Revenue Service, or the IRS, with respect to the statements made and the conclusions reached in the following summary, and there can be no assurance that the IRS will agree with such statements and conclusions.
This summary does not address the tax considerations arising under the laws of any state, local, non-U.S. or other jurisdiction or, excepts as provided below, under U.S. federal estate and gift tax laws, except to the limited extent set forth below, and is limited to investors who will hold our common stock as a capital asset for tax purposes. This summary does not address the potential application of the federal tax on net investment income or any tax considerations applicable to an investor’s particular circumstances or to investors that may be subject to special rules, such as:
|
• |
banks, insurance companies or other financial institutions; |
|
• |
persons subject to the alternative minimum tax; |
|
• |
tax-exempt organizations; |
|
• |
controlled foreign corporations, passive foreign investment companies and corporations that accumulate earnings to avoid U.S. federal income tax; |
|
• |
dealers in securities or currencies; |
|
• |
traders in securities that elect to use a mark-to-market method of accounting for their securities holdings; |
|
• |
persons that own, or are deemed to own, more than 5% of our capital stock (except to the extent specifically set forth below); |
|
• |
certain former citizens or long-term residents of the United States; |
|
• |
persons who hold our common stock as a position in a hedging transaction, “straddle,” “conversion transaction” or other risk reduction transaction; or |
|
• |
persons deemed to sell our common stock under the constructive sale provisions of the Code. |
In addition, if a partnership (including any entity classified as a partnership for U.S. federal income tax purposes) holds our common stock, the tax treatment of a partner generally will depend on the status of the partner and upon the activities of the partnership. Accordingly, partnerships that hold our common stock, and partners in such partnerships, should consult their tax advisors.
You are urged to consult your tax advisor with respect to the application of the U.S. federal income tax laws to your particular situation, as well as any tax consequences of the purchase, ownership and disposition of our common stock arising under other U.S. federal tax rules or under the laws of any state, local, non-U.S. or other taxing jurisdiction or under any applicable tax treaty.
Non-U.S. holder defined
For purposes of this discussion, you are a non-U.S. holder if you are a holder other than a partnership (or other entity treated as a partnership for U.S. federal income tax purposes) and not a (1) U.S. citizen or U.S. resident alien, (2) a corporation or other entity taxable as a corporation for U.S. federal income tax purposes that was created or organized in or under the laws of the United States, any state thereof or the District of Columbia, (3) an estate whose income is subject to U.S. federal income taxation regardless of its source, or (4) a trust that either is subject to the supervision of a court within the United States and has one or more U.S. persons with authority to control all of its substantial decisions, or has a valid election in effect under applicable Treasury regulations to be treated as a U.S. person.
-79-
Distributions on common stock
We have not made any distributions on our common stock. However, if we make distributions on our common stock, these distributions generally will constitute dividends for U.S. tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. To the extent these distributions exceed both our current and our accumulated earnings and profits, they will constitute a return of capital and will first reduce your basis in our common stock, but not below zero, and then will be treated as gain from the sale of stock as described below.
Subject to the discussion below regarding backup withholding and recent legislation relating to foreign accounts, any dividend paid to you generally will be subject to U.S. withholding either at a rate of 30% of the gross amount of the dividend or such lower rate as may be specified by an applicable income tax treaty. In order to receive a reduced treaty rate, you must provide the applicable withholding agent with an IRS Form W-8BEN or other appropriate version of IRS Form W-8 certifying qualification for the reduced rate. If you are eligible for a reduced rate of withholding pursuant to an income tax treaty, you may obtain a refund of any excess amounts withheld by filing an appropriate claim for refund with the IRS. If you hold our common stock through a financial institution or other agent acting on your behalf, you will be required to provide appropriate documentation to the agent, which then will be required to provide certification to the applicable withholding agent, either directly or through other intermediaries.
Dividends received by you that are effectively connected with your conduct of a U.S. trade or business (and, if an income tax treaty applies, attributable to a permanent establishment maintained by you in the United States) are exempt from such withholding tax. In order to claim this exemption, you must provide the applicable withholding agent with an IRS Form W-8ECI or other applicable IRS Form W-8 properly certifying such exemption. Such effectively connected dividends, although not subject to withholding tax, are taxed at the same graduated U.S. federal income tax rates applicable to U.S. persons, net of certain deductions and credits. In addition, if you are a corporate non-U.S. holder, dividends you receive that are effectively connected with your conduct of a U.S. trade or business may also be subject to a branch profits tax at a rate of 30% or such lower rate as may be specified by an applicable income tax treaty.
Gain on disposition of common stock
Subject to the discussion below regarding backup withholding and recent legislation relating to foreign accounts, you generally will not be subject to U.S. federal income tax on any gain realized upon the sale or other disposition of our common stock unless:
|
• |
the gain is effectively connected with your conduct of a U.S. trade or business (and, if an income tax treaty applies, the gain is attributable to a permanent establishment maintained by you in the United States); |
|
• |
you are an individual who is present in the United States for a period or periods aggregating 183 days or more during the calendar year in which the sale or disposition occurs and certain other conditions are met; or |
|
• |
our common stock constitutes a U.S. real property interest by reason of our status as a “United States real property holding corporation,” or USRPHC, for U.S. federal income tax purposes at any time within the shorter of the five-year period preceding your disposition of our common stock and your holding period for our common stock. |
If you are a non-U.S. holder described in the first bullet above, you will be required to pay tax on the net gain derived from the sale at regular graduated U.S. federal income tax rates applicable to U.S. persons (net of certain deductions and credits), and if you are a corporate non-U.S. holder, you may also be subject to branch profits tax at a rate of 30% or such lower rate as may be specified by an applicable income tax treaty. If you are a non-U.S. holder described in the second bullet above, you will be required to pay a flat 30% tax on the gain derived from the sale, which tax may be offset by U.S. source capital losses (even though you are not considered a resident of the United States).
We believe that we are not currently and will not become a USRPHC. However, because the determination of whether we are a USRPHC depends on the fair market value of our U.S. real property relative to the fair market value of our other business assets, there can be no assurance that we will not become a USRPHC in the future. Even if we become a USRPHC, however, as long as our common stock is regularly traded on an established securities market, our common stock will be treated as a U.S. real property interest only if you actually or constructively hold more than 5% of such regularly traded common stock at any time during the shorter of the five-year period preceding your disposition of our common stock or your holding period for our common stock.
Federal estate tax
Our common stock beneficially owned by an individual who is not a citizen or resident of the United States (as defined for U.S. federal estate tax purposes) at the time of death, generally will be includable in the decedent’s gross estate for U.S. federal estate tax purposes, unless an applicable estate tax treaty provides otherwise.
-80-
Backup withholding and information reporting
Generally, we must report annually to the IRS the amount of dividends paid to you, your name and address, and the amount of tax withheld, if any. A similar report will be sent to you. Pursuant to applicable income tax treaties or other agreements, the IRS may make these reports available to tax authorities in your country of residence.
Payments of dividends on, or the gross proceeds of a disposition of, our common stock may be subject to additional information reporting and backup withholding at a current rate of 28% unless you establish an exemption, for example by properly certifying your non-U.S. status on an IRS Form W-8BEN or another appropriate version of IRS Form W-8. Notwithstanding the foregoing, backup withholding and information reporting may apply if either we or our paying agent has actual knowledge, or reason to know, that you are a U.S. person.
Backup withholding is not an additional tax. Any amounts withheld from a payment to you under the backup withholding rules will be allowed as a credit against your U.S. federal income tax liability and may entitle you to a refund, provided that the required information or returns are furnished to the IRS in a timely manner.
Foreign accounts
The Foreign Account Tax Compliance Act, or FATCA, generally imposes a U.S. federal withholding tax of 30% on dividends on, and the gross proceeds of a disposition of, our common stock paid to a “foreign financial institution” (as specifically defined for this purpose) unless such institution enters into an agreement with the U.S. government to withhold on certain payments and to collect and provide to the U.S. tax authorities substantial information regarding U.S. account holders of such institution (which may include certain equity and debt holders of such institution, as well as certain account holders that are foreign entities with U.S. owners) or otherwise establishes an exemption. FATCA will generally impose a U.S. federal withholding tax of 30% on dividends and the gross proceeds of a disposition of our common stock to a non-financial foreign entity unless such entity provides the withholding agent with a certification identifying certain substantial direct and indirect U.S. owners of the entity, certifies that there are none or otherwise establishes an exemption. This withholding obligation under FATCA with respect to dividends on our common stock began July 1, 2014 and with respect to the gross proceeds of a sale or other disposition of our common stock will not begin until January 1, 2017. Under certain circumstances, a non-U.S. holder might be eligible for refunds or credits of such taxes. An intergovernmental agreement between the United States and an applicable foreign country may modify the requirements described in this paragraph. Prospective investors are encouraged to consult with their tax advisors regarding the possible implications of FATCA on their investment in our common stock.
Each prospective investor should consult its tax advisor regarding the particular U.S. federal, state and local and non-U.S. tax consequences of purchasing, holding and disposing of our common stock, including the consequences of any proposed change in applicable laws.
-81-
We, the selling stockholders and the underwriters named below have entered into an underwriting agreement with respect to the shares being offered. Subject to certain conditions, each underwriter has severally agreed to purchase the number of shares indicated in the following table. J.P. Morgan Securities LLC is acting as book-running manager of the offering and as representative of the underwriters.
|
Underwriter |
|
Number of |
|
|
|
J.P. Morgan Securities LLC |
|
|
|
|
|
William Blair & Company, L.L.C |
|
|
|
|
|
Leerink Partners, LLC |
|
|
|
|
|
Needham & Company, LLC |
|
|
|
|
|
Total |
|
|
|
|
The underwriters are committed to take and pay for all of the shares being offered, if any are taken.
The underwriters propose to offer the shares of common stock directly to the public at the offering price set forth on the cover page of this prospectus and to certain dealers at that price less a concession not in excess of $ per share. After the initial offering of the shares, the offering price and other selling terms may be changed by the underwriters. Sales of shares made outside of the United States may be made by affiliates of the underwriters. The representatives have advised us that the underwriters do not intend to confirm discretionary sales in excess of 5% of the common shares offered in this offering.
The underwriting fee is equal to the public offering price per share of common stock less the amount paid by the underwriters to the selling stockholders per share of common stock. The underwriting fee is $ per share. The following tables show the per share and total underwriting discounts and commissions to be paid by the selling stockholders to the underwriters.
|
|
|
Shares being offered |
|
Optional shares |
|
|
|
Per share |
|
$ |
|
|
|
|
|
Total |
|
$ |
|
|
|
|
We estimate that the total expenses of this offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding the underwriting discounts and commissions, will be approximately $ . We have agreed to reimburse the underwriters for certain expenses, including up to an aggregate of $ in connection with the clearance of this offering with the Financial Industry Regulatory Authority, as set forth in the underwriting agreement.
A prospectus in electronic format may be made available on the websites maintained by one or more underwriters, or selling group members, if any, participating in the offering. The underwriters may agree to allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the representatives to underwriters and selling group members that may make Internet distributions on the same basis as other allocations.
We, all of our directors and executive officers and the selling stockholders signed lock-up agreements under which, subject to certain exceptions, each agreed not to sell, transfer or dispose of or hedge, directly or indirectly, any shares of our common stock or any securities convertible into or exercisable or exchangeable for shares of our common stock, in each case without the prior written consent of J.P. Morgan Securities LLC, for a period of 60 days after the date of this prospectus. Subject to certain limitations, the restrictions applicable to us do not apply to (A) any shares of common stock issued upon the exercise of options or warrants or the conversion of a security outstanding on the date hereof and described in this prospectus, (B) the grant of options or the issuance of shares of common stock by the Company to employees, officers, directors, advisors or consultants of the Company pursuant to employee benefit plans in effect on the date hereof and described in this prospectus, (C) the filing by the Company of a registration statement with the SEC on Form S-8 in respect of any shares issued under or the grant of any award pursuant to an employee benefit plan in effect on the date hereof and described in this prospectus or (D) the sale or issuance of or entry into an agreement to sell or issue shares of common stock or securities convertible into or exercisable or exchangeable for common stock in connection with any mergers, acquisition of securities, businesses, proper or other assets, joint ventures, strategic alliances, equipment leasing arrangements or debt financing. Also subject to certain limitations, the restrictions applicable to our directors and executive officers and the selling stockholders do not apply to (A) the shares of common stock to be sold by the selling stockholders in this offering, (B) transfers of shares of common stock or any securities convertible into or exercisable or exchangeable for common stock (i) by bona fide gift, will or intestacy, or (ii) to an immediate family member or to a trust formed for the benefit of an immediate family member, (C) if the signatory is a trust, to a trustee or beneficiary of the trust, (D) if the signatory is a corporation, partnership or other
-82-
business entity, transfers of shares of common stock (i) to another corporation, partnership or other business entity that controls, is controlled by or is under common control with the signatory or (ii) as part of a disposition, transfer or distribution without consideration by the signatory to its equity holders, (E) sales of shares of common stock or other securities acquired in open market transactions after the completion of this offering, (F) the transfer of shares of common stock or any security convertible into common stock to the Company upon a vesting event of the Company’s securities or upon the exercise of options or warrants to purchase the Company’s securities, in each case on a “cashless” or “net exercise” basis or to cover tax withholding obligations of the signatory in connection with such vesting or exercise, (G) sales or transfers of common stock made pursuant to a trading plan pursuant to Rule 10b5-1 under the Exchange Act (“Rule 10b5-1”) that has been entered into by the signatory prior to the date of the lock-up agreement, (H) the establishment of a trading plan pursuant to Rule 10b5-1 under the Exchange Act for the transfer of shares of common stock, or (I) the transfer of shares of common stock or any security convertible into or exercisable or exchangeable for common stock pursuant to a bona fide third party tender offer, merger, consolidation or other similar transaction made to all holders of the common stock involving a change of control of the Company.
We and the selling stockholders have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act of 1933.
Our common stock is listed on the NASDAQ Global Select Market under the symbol “INGN.”
In connection with this offering, the underwriters may engage in stabilizing transactions, which involves making bids for, purchasing and selling shares of common stock in the open market for the purpose of preventing or retarding a decline in the market price of the common stock while this offering is in progress. These stabilizing transactions may include making short sales of the common stock, which involves the sale by the underwriters of a greater number of shares of common stock than they are required to purchase in this offering, and purchasing shares of common stock on the open market to cover positions created by short sales. To the extent that the underwriters create a naked short position, they will purchase shares in the open market to cover the position.
The underwriters have advised us that, pursuant to Regulation M of the Securities Act of 1933, they may also engage in other activities that stabilize, maintain or otherwise affect the price of the common stock, including the imposition of penalty bids. This means that if the representatives of the underwriters purchase common stock in the open market in stabilizing transactions or to cover short sales, the representatives can require the underwriters that sold those shares as part of this offering to repay the underwriting discount received by them.
These activities may have the effect of raising or maintaining the market price of the common stock or preventing or retarding a decline in the market price of the common stock, and, as a result, the price of the common stock may be higher than the price that otherwise might exist in the open market. If the underwriters commence these activities, they may discontinue them at any time. The underwriters may carry out these transactions on the NASDAQ Global Select Market, in the over-the-counter market or otherwise.
Relationships with underwriters
The underwriters and their respective affiliates are full-service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing, and brokerage activities. The underwriters and their affiliates have not, during the 180-day period preceding the date of the initial filing of the Registration Statement on Form S-1 of which this prospectus forms a part, but may, in the future, provide from time to time certain commercial banking, financial advisory, investment banking and other services for us and such affiliates in the ordinary course of their business, for which they may receive customary fees and commissions. Except as disclosed in this prospectus, we have no present arrangements with any of the underwriters for any further services. In addition, from time to time, certain of the underwriters and their affiliates may effect transactions for their own account or the account of customers, and hold on behalf of themselves or their customers, long or short positions in our debt or equity securities or loans, and may do so in the future.
Selling restrictions outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The shares of common stock offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
-83-
This document is only being distributed to and is only directed at (i) persons who are outside the United Kingdom or (ii) to investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, referred to as the Order, or (iii) high net worth entities, and other persons to whom it may lawfully be communicated, falling with Article 49(2)(a) to (d) of the Order, all such persons together being referred to as relevant persons. The shares of common stock are only available to, and any invitation, offer or agreement to subscribe, purchase or otherwise acquire such securities will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of its contents.
Notice to prospective investors in the European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive, each referred to as a Relevant Member State, from and including the date, or Relevant Implementation Date, on which the European Union Prospectus Directive, or EU Prospectus Directive, was implemented in that Relevant Member State, an offer of shares of common stock described in this prospectus may not be made to the public in that Relevant Member State prior to the publication of a prospectus in relation to the shares which has been approved by the competent authority in that Relevant Member State or, where appropriate, approved in another Relevant Member State and notified to the competent authority in that Relevant Member State, all in accordance with the EU Prospectus Directive, except that, with effect from and including the Relevant Implementation Date, an offer of securities described in this prospectus may be made to the public in that Relevant Member State at any time:
|
• |
to any legal entity which is a qualified investor as defined under the EU Prospectus Directive; |
|
• |
to fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150 natural or legal persons (other than qualified investors as defined in the EU Prospectus Directive), as permitted under the EU Prospectus Directive, subject to obtaining the prior consent of J.P. Morgan Securities LLC for any such offer; or |
|
• |
in any other circumstances falling within Article 3(2) of the EU Prospectus Directive, provided that no such offer of securities described in this prospectus shall result in a requirement for the publication by us of a prospectus pursuant to Article 3 of the EU Prospectus Directive. |
For the purposes of this provision, the expression an “offer of securities to the public” in relation to any securities in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the shares of common stock to be offered so as to enable an investor to decide to purchase or subscribe for the shares, as the same may be varied in that Member State by any measure implementing the EU Prospectus Directive in that Member State. The expression “EU Prospectus Directive” means Directive 2003/71/EC (and any amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State) and includes any relevant implementing measure in each Relevant Member State, and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
Notice to prospective investors in the United Kingdom
Each underwriter has represented and agreed that:
(a) it has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in investment activity (within the meaning of Section 21 of the FSMA) received by it in connection with the issue or sale of the shares in circumstances in which Section 21(1) of the FSMA does not apply to the Issuer; and
(b) it has complied and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the shares in, from or otherwise involving the United Kingdom.
Notice to prospective investors in Switzerland
The shares may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange, or the SIX, or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this prospectus nor any other offering or marketing material relating to the shares or the offering may be publicly distributed or otherwise made publicly available in Switzerland. Neither this prospectus nor any other offering or marketing material relating to the offering, the Company, the shares have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of shares will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA, and the offer of shares has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes, or the CISA. The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of shares.
-84-
Notice to prospective investors in Hong Kong
The shares may not be offered or sold by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), or (ii) to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a “prospectus” within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), and no advertisement, invitation or document relating to the shares may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to shares which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder.
Notice to prospective investors in Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore, or the SFA, (ii) to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the shares are subscribed or purchased under Section 275 by a relevant person which is: (a) a corporation (which is not an accredited investor) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or (b) a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary is an accredited investor, shares, debentures and units of shares and debentures of that corporation or the beneficiaries’ rights and interest in that trust shall not be transferable for 6 months after that corporation or that trust has acquired the shares under Section 275 except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA; (2) where no consideration is given for the transfer; or (3) by operation of law.
Notice to prospective investors in Japan
The securities have not been and will not be registered under the Financial Instruments and Exchange Law of Japan (the Financial Instruments and Exchange Law) and each underwriter has agreed that it will not offer or sell any securities, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan (which term as used herein means any person resident in Japan, including any corporation or other entity organized under the laws of Japan), or to others for re-offering or resale, directly or indirectly, in Japan or to a resident of Japan, except pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the Financial Instruments and Exchange Law and any other applicable laws, regulations and ministerial guidelines of Japan.
-85-
The validity of the shares of common stock offered hereby will be passed upon for us by Wilson Sonsini Goodrich & Rosati, Professional Corporation, Los Angeles, California. Latham & Watkins LLP, Costa Mesa, California is representing the underwriters.
The financial statements and schedule as of and for the years ended December 31, 2013 and 2012 incorporated by reference in this Registration Statement have been so incorporated in reliance on the report of BDO USA, LLP, an independent registered public accounting firm, incorporated herein by reference given on the authority of said firm as experts in auditing and accounting.
The financial statements and schedule for the year ended December 31, 2011 incorporated by reference in this Registration Statement have been so incorporated in reliance on the report of Macias Gini & O’Connell LLP, an independent registered public accounting firm, incorporated herein by reference, given on the authority of said firm as experts in auditing and accounting.
Where you can find additional information
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of common stock offered by this prospectus. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement, some of which is contained in exhibits to the registration statement as permitted by the rules and regulations of the SEC. For further information with respect to us and our common stock, we refer you to the registration statement, including the exhibits filed as a part of the registration statement. Statements contained in this prospectus concerning the contents of any contract or any other document is not necessarily complete. If a contract or document has been filed as an exhibit to the registration statement, please see the copy of the contract or document that has been filed. Each statement in this prospectus relating to a contract or document filed as an exhibit is qualified in all respects by the filed exhibit. You may obtain copies of this information by mail from the Public Reference Section of the SEC, 100 F Street, N.E., Room 1580, Washington, D.C. 20549, at prescribed rates. You may obtain information on the operation of the public reference rooms by calling the SEC at 1-800-SEC-0330. The SEC also maintains an internet website that contains reports, proxy statements, and other information about issuers, like us, that file electronically with the SEC. The address of that website is www.sec.gov.
We are subject to the information and reporting requirements of the Securities Exchange Act of 1934 and, in accordance with this law, are required to file periodic reports, proxy statements, and other information with the SEC. These periodic reports, proxy statements, and other information are available for inspection and copying at the SEC’s public reference facilities and the website of the SEC referred to above. We also maintain a website at www.inogen.com. You may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. Information contained on our website is not a part of this prospectus and the inclusion of our website address in this prospectus is an inactive textual reference only.
-86-
The SEC allows us to “incorporate by reference” information in this prospectus that we have filed with it. This means that we can disclose important information to you by referring you to another document already on file with the SEC.
This prospectus incorporates by reference the documents listed below that we have previously filed with the SEC (excluding any document, or portion thereof, to the extent disclosure is furnished and not filed):
|
• |
our Annual Report on Form 10-K for the fiscal year ended December 31, 2013, filed with the SEC on April 1, 2014; |
|
• |
our Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2014, filed with the SEC on May 13, 2014; |
|
• |
our Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2014, filed with the SEC on August 12, 2014; and |
|
• |
our Current Reports on Form 8-K filed with the SEC on February 25, 2014, March 21, 2014, and August 7, 2014. |
Any statements contained in a document incorporated by reference into this prospectus will be deemed to be modified or superseded for the purposes of this prospectus to the extent that a later statement contained in this prospectus or in any other document incorporated by reference into this prospectus modifies or supersedes the earlier statement. Any statement so modified or superseded will not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
We will provide to each person, including any beneficial owners, to whom a prospectus is delivered, a copy of the reports and documents that have been incorporated by reference into this prospectus, at no cost. Any such request may be made by writing or telephoning us at the following address or phone number:
Inogen, Inc.
326 Bollay Drive
Goleta, California 93117
Telephone: (805) 562-0500
These documents can also be requested through, and are available in, the Investors section of our website, which is located at www.inogen.com, or as described in “Where you can find additional information” above. The reference to our website address does not constitute incorporation by reference of the information contained on our website.
-87-


Part II
Information not required in the prospectus
Item 13. Other expenses of issuance and distribution.
The following table sets forth the estimated expenses payable by us in connection with the sale and distribution of the securities registered herby, other than underwriting discounts and commissions. All amounts are estimates except for the SEC registration fee and the Financial Industry Regulatory Authority filing fee.
|
|
|
Amount to be paid |
|
|
|
SEC registration fee |
|
$ |
|
|
|
FINRA filing fee |
|
|
|
|
|
Exchange listing fee |
|
|
|
|
|
Printing and engraving expenses |
|
|
|
|
|
Legal fees and expenses |
|
|
|
|
|
Accounting fees and expenses |
|
|
|
|
|
Transfer agent and registrar fees and expenses |
|
|
|
|
|
Miscellaneous |
|
|
|
|
|
Total |
|
$ |
|
|
Item 14. Indemnification of directors and officers.
Section 145 of the Delaware General Corporation Law, or DGCL, empowers a corporation to indemnify its directors and officers and to purchase insurance with respect to liability arising out of their capacity or status as directors and officers, provided that the person acted in good faith and in a manner the person reasonably believed to be in its best interests, and, with respect to any criminal action, had no reasonable cause to believe the person’s actions were unlawful. The DGCL further provides that the indemnification permitted thereunder shall not be deemed exclusive of any other rights to which the directors and officers may be entitled under the corporation’s bylaws, any agreement, a vote of stockholders or otherwise. The certificate of incorporation of the registrant provides for the indemnification of the registrant’s directors and officers to the fullest extent permitted under the DGCL. In addition, the bylaws of the registrant require the registrant to fully indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding (whether civil, criminal, administrative or investigative) by reason of the fact that such person is or was a director, or officer of the registrant, or is or was a director or officer of the registrant serving at the registrant’s request as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorney’s fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, to the fullest extent permitted by applicable law.
Section 102(b)(7) of the DGCL permits a corporation to provide in its certificate of incorporation that a director of the corporation shall not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director, except (1) for any breach of the director’s duty of loyalty to the corporation or its stockholders, (2) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (3) for payments of unlawful dividends or unlawful stock repurchases or redemptions or (4) for any transaction from which the director derived an improper personal benefit. The registrant’s certificate of incorporation provides that the registrant’s directors shall not be personally liable to it or its stockholders for monetary damages for breach of fiduciary duty as a director and that if the DGCL is amended to authorize corporate action further eliminating or limiting the personal liability of directors, then the liability of the registrant’s directors shall be eliminated or limited to the fullest extent permitted by the Delaware General Corporation Law, as so amended.
Section 174 of the DGCL provides, among other things, that a director who willfully or negligently approves of an unlawful payment of dividends or an unlawful stock purchase or redemption may be held liable for such actions. A director who was either absent when the unlawful actions were approved, or dissented at the time, may avoid liability by causing his or her dissent to such actions to be entered in the books containing minutes of the meetings of board of directors at the time such action occurred or immediately after such absent director receives notice of the unlawful acts.
As permitted by the DGCL, the registrant has entered into separate indemnification agreements with each of the registrant’s directors and certain of the registrant’s officers which require the registrant, among other things, to indemnify them against certain liabilities which may arise by reason of their status as directors, officers or certain other employees.
The registrant expects to obtain and maintain insurance policies under which its directors and officers are insured, within the limits and subject to the limitations of those policies, against certain expenses in connection with the defense of, and certain liabilities which might be imposed as a result of, actions, suits or proceedings to which they are parties by reason of being or having been directors or
II-1
officers. The coverage provided by these policies may apply whether or not the registrant would have the power to indemnify such person against such liability under the provisions of the DGCL.
These indemnification provisions and the indemnification agreements entered into between the registrant and the registrant’s officers and directors may be sufficiently broad to permit indemnification of the registrant’s officers and directors for liabilities (including reimbursement of expenses incurred) arising under the Securities Act of 1933, as amended, or Securities Act.
The underwriting agreement between the registrant and the underwriters filed as Exhibit 1.1 to this registration statement provides for the indemnification by the underwriters of the registrant’s directors and officers and certain controlling persons against specified liabilities, including liabilities under the Securities Act with respect to information provided by the underwriters specifically for inclusion in the registration statement.
Item 15. Recent sales of unregistered securities.
The following list sets forth information regarding all unregistered securities sold or granted by us since January 1, 2011. No underwriters were involved in the sales and the certificates representing the securities sold and issued contain legends restricting transfer of the securities without registration under the Securities Act or an applicable exemption from registration.
(a) In March 2012, the registrant sold an aggregate of 2,840,260 shares of its series G convertible preferred stock at $7.0416 per share for aggregate proceeds of approximately $20,000,000 to a total of eight accredited investors.
(b) From January 1, 2011 through December 7, 2011, the registrant granted to certain of its employees, consultants, directors and other service providers under the registrant’s 2002 Stock Incentive Plan options to purchase an aggregate of 158,841 shares of its common stock at an exercise price of $0.75 per share.
(c) From March 28, 2012 through February 18, 2014, the registrant granted to certain of its employees, consultants, directors and other service providers under the registrant’s 2012 Equity Incentive Plan options to purchase an aggregate of 964,922 shares of its common stock at exercise prices ranging from $0.81 to $8.37 per share.
(d) From January 1, 2011 through February 18, 2014, the registrant issued and sold an aggregate of 17,079 shares of its common stock upon the exercise of options issued to certain employees, directors and consultants under the registrant’s 2002 Stock Incentive Plan at exercise prices ranging from $0.60 to $8.70, for aggregate consideration of approximately $22,000.
(e) On March 4, 2011, the registrant issued 2,554 shares of its series C convertible preferred stock upon exercise of warrants at an exercise price of $17.58 per share for aggregate proceeds of approximately $45,000.
(f) On February 28, 2012, the registrant issued 17,386 shares of its common stock upon exercise of warrants at an exercise price of $0.30 per share for aggregate proceeds of approximately $5,000.
(g) On April 18, 2012, the registrant issued 8,408 shares of its series C convertible preferred stock upon exercise of warrants at an exercise price of $17.58 per share for aggregate proceeds of approximately $148,000.
(h) On April 18, 2012, the registrant issued 2,429 shares of its series B convertible preferred stock upon exercise of a warrant at an exercise price of $11.88 per share for aggregate proceeds of approximately $29,000.
(i) On December 27, 2012, the registrant issued 13,647 shares of its series C convertible preferred stock upon exercise of warrants at an exercise price of $17.58 per share for aggregate proceeds of approximately $240,000.
(j) On February 14, 2013, the registrant issued 19,976 shares of its series D convertible preferred stock upon exercise of warrants at an exercise price of $21.90 per share for aggregate proceeds of approximately $437,000.
(k) On February 28, 2013, the registrant issued 19,539 shares of its series D convertible preferred upon exercise of warrants at an exercise price of $21.90 per share for aggregate proceeds of approximately $428,000.
(l) On May 20, 2013, the registrant issued 7,989 shares of its series D convertible preferred stock upon exercise of warrants at an exercise price of $21.90 per share for aggregate proceeds of approximately $175,000.
(m) On May 23, 2013, the registrant issued 2,951 shares of its series D convertible preferred stock upon exercise of a warrant at an exercise price of $21.90 per share for aggregate proceeds of approximately $65,000.
(n) On June 21, 2013, the registrant issued 5,706 shares of its series D convertible preferred stock upon exercise of warrants at an exercise price of $21.90 per share for aggregate proceeds of approximately $125,000.
(o) On July 3, 2013, the registrant issued 3,685 shares of its series D convertible preferred stock upon exercise of a warrant at an exercise price of $21.90 per share for aggregate proceeds of approximately $81,000.
(p) On August 28, 2013, the registrant issued 22,830 shares of its series D convertible preferred stock upon exercise of warrants at an exercise price of $21.90 per share for aggregate proceeds of approximately $500,000.
II-2
(q) On September 5, 2013, the registrant issued 2,853 shares of its series D convertible preferred stock upon exercise of a warrant at an exercise price of $21.90 per share for aggregate proceeds of approximately $62,000.
(r) On October 28, 2013, the registrant issued 372 shares of its series D convertible preferred stock upon exercise of a warrant at an exercise price of $21.90 per share for aggregate proceeds of approximately $8,000.
(s) On January 6, 2014, the registrant issued 2,045 shares of its series C convertible preferred stock upon exercise of warrants at an exercise price of $17.58 per share for aggregate proceeds of approximately $36,000.
(t) On January 6, 2014, the registrant issued 7,649 shares of its common stock upon exercise of warrants at an exercise price of $0.30 per share for aggregate proceeds of approximately $2,000.
(u) On January 15, 2014, the registrant issued 8,951 shares of its series C convertible preferred stock upon exercise of warrants at an exercise price of $17.58 per share for aggregate proceeds of approximately $157,000.
(v) On January 15, 2014, the registrant issued 49,436 shares of its common stock upon exercise of warrants at an exercise price of $0.30 per share for aggregate proceeds of approximately $15,000.
(w) On January 17, 2014, the registrant issued 11,415 shares of its series D convertible preferred stock upon exercise of warrants at an exercise price of $21.90 per share for aggregate proceeds of approximately $250,000.
(x) On January 21, 2014, the registrant issued 98 shares of its series C convertible preferred stock upon exercise of warrants at an exercise price of $17.58 per share for aggregate proceeds of approximately $2,000.
(y) On January 27, 2014, the registrant issued 15,231 shares of its common stock upon exercise of warrants at an exercise price of $0.30 per share for aggregate proceeds of approximately $4,500.
(z) On February 14, 2014, the registrant issued 228 shares of common stock upon the cashless exercise of one outstanding warrant based on a weighted average exercise price of $17.58 per share.
(aa) On April 22, 2014, the registrant issued 4,053 shares of common stock upon the cashless exercise of seven outstanding warrants based on a weighted average exercise price of $9.61.
(bb) On September 4, 2014, the registrant issued 15,987 shares of common stock upon the cashless exercise of six outstanding warrants based on a weighted average exercise price of $0.30 per share.
Unless otherwise indicated, the offers, sales and issuances of the securities described in Items 15(a) and 15(e) through (bb) were exempt from registration under the Securities Act under Section 4(2) of the Securities Act as transactions by an issuer not involving a public offering. The recipients of securities in each of these transactions acquired the securities for investment only and not with a view to or for sale in connection with any distribution thereof and appropriate legends were affixed to the securities issued in these transactions. Each of the recipients of securities in these transactions was an accredited person and had adequate access, through employment, business or other relationships, to information about the registrant.
The offers, sales and issuances of the securities described in Items 15(b), 15(c) and 15(d) were exempt from registration under the Section 4(2) of the Securities Act and/or Rule 701 of the Securities Act.
II-3
Item 16. Exhibits and financial statement schedules.
(a) Exhibits.
We have filed the exhibits listed on the accompanying Exhibit Index of this Registration Statement.
(b) Financial statement schedules.
Schedules have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
Item 17. Undertakings.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned hereby undertakes that:
|
(1) |
For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
|
(2) |
For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-4
Signatures
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Goleta, State of California, on October 14, 2014.
|
INOGEN, INC. |
||
|
|
|
|
|
By: |
|
/s/ Raymond Huggenberger |
|
|
|
Raymond Huggenberger |
|
|
|
President and Chief Executive Officer |
Power of attorney
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Raymond Huggenberger and Alison Bauerlein as his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities (including his capacity as a director and/or officer of Inogen, Inc.) to sign any or all amendments (including post-effective amendments) to this registration statement and any and all additional registration statements pursuant to Rule 462(b) of the Securities Act of 1933, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as they, he or she might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agents or any of them, or their, his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated:
|
Signature |
|
Title |
|
Date |
|
/s/ Raymond Huggenberger |
|
President, Chief Executive Officer and |
|
October 14, 2014 |
|
Raymond Huggenberger |
|
Director (Principal Executive Officer) |
|
|
|
|
|
|
|
|
|
/s/ Alison Bauerlein |
|
Chief Financial Officer |
|
October 14, 2014 |
|
Alison Bauerlein |
|
(Principal Accounting and Financial Officer) |
|
|
|
|
|
|
|
|
|
/s/ Heath Lukatch, Ph.D. |
|
Chairman of the Board |
|
October 14, 2014 |
|
Heath Lukatch, Ph.D. |
|
|
|
|
|
|
|
|
|
|
|
/s/ Benjamin Anderson-Ray |
|
Director |
|
October 14, 2014 |
|
Benjamin Anderson-Ray |
|
|
|
|
|
|
|
|
|
|
|
/s/ Heather Rider |
|
Director |
|
October 14, 2014 |
|
Heather Rider |
|
|
|
|
|
|
|
|
|
|
|
/s/ Loren McFarland |
|
Director |
|
October 14, 2014 |
|
Loren McFarland |
|
|
|
|
|
|
|
|
|
|
|
/s/ Timothy Petersen |
|
Director |
|
October 14, 2014 |
|
Timothy Petersen |
|
|
|
|
II-5
EXHIBIT INDEX
|
Exhibit |
|
|
|
Incorporated by Reference |
|||||||
|
|
Description of Exhibit |
|
Form |
|
File No. |
|
Exhibit |
|
Filing Date |
||
|
|
|
|
|
|
|
|
|||||
|
1.1* |
|
Form of Underwriting Agreement. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
3.1 |
|
Thirteenth Amended and Restated Certificate of Incorporation of the Registrant. |
|
S-1 |
|
333-192605 |
|
3.2 |
|
11/27/13 |
|
|
|
|
|
|
|
|
|
|||||
|
3.2 |
|
Amended and Restated Bylaws of the Registrant. |
|
S-1 |
|
333-192605 |
|
3.3 |
|
11/27/13 |
|
|
|
|
|
|
|
|
|
|||||
|
4.1 |
|
Specimen Common Stock Certificate of the Registrant. |
|
S-1/A |
|
333-192605 |
|
4.1 |
|
01/16/14 |
|
|
|
|
|
|
|
|
|
|||||
|
4.2 |
|
Ninth Amended and Restated Investors’ Rights Agreement, dated March 12, 2012, by and among the Registrant and the investors named therein, as amended. |
|
S-1/A |
|
333-192605 |
|
4.2 |
|
01/16/14 |
|
|
|
|
|
|
|
|
|
|||||
|
4.3 |
|
Form of Warrant to Purchase Common Stock issued in connection with the Registrant’s 2007 convertible note financing. |
|
S-1 |
|
333-192605 |
|
4.3 |
|
11/27/13 |
|
|
|
|
|
|
|
|
|
|||||
|
5.1* |
|
Opinion of Wilson Sonsini Goodrich & Rosati, Professional Corporation. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
10.1+ |
|
Form of Director and Executive Officer Indemnification Agreement. |
|
S-1 |
|
333-192605 |
|
10.1 |
|
11/27/13 |
|
|
|
|
|
|
|
|
|
|||||
|
10.2+ |
|
2002 Stock Plan, as amended. |
|
S-1 |
|
333-192605 |
|
10.2 |
|
11/27/13 |
|
|
|
|
|
|
|
|
|
|||||
|
10.3+ |
|
Form of Notice of Stock Option Grant and Stock Option Agreement under the 2002 Stock Plan, as amended. |
|
S-1 |
|
333-192605 |
|
10.3 |
|
11/27/13 |
|
|
|
|
|
|
|
|
|
|||||
|
10.4+ |
|
2012 Equity Incentive Plan, as amended. |
|
S-1 |
|
333-192605 |
|
10.4 |
|
11/27/13 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.5+ |
|
Form of Stock Option Agreement under the 2012 Equity Incentive Plan. |
|
S-1 |
|
333-192605 |
|
10.5 |
|
11/27/13 |
|
|
|
|
|
|
|
|
|
|||||
|
10.6+ |
|
2014 Equity Incentive Plan. |
|
S-1/A |
|
333-192605 |
|
10.6 |
|
01/28/14 |
|
|
|
|
|
|
|
|
|
|||||
|
10.7+ |
|
Form Agreements under the 2014 Equity Incentive Plan. |
|
S-1/A |
|
333-192605 |
|
10.7 |
|
01/28/14 |
|
|
|
|
|
|
|
|
||||||
|
10.8+ |
|
2014 Employee Stock Purchase Plan. |
|
S-1/A |
|
333-192605 |
|
10.8 |
|
01/28/14 |
|
|
|
|
|
|
|
|
|
|||||
|
10.9+ |
|
Executive Incentive Compensation Plan. |
|
S-1 |
|
333-192605 |
|
10.9 |
|
11/27/13 |
|
|
|
|
|
|
|
|
|
|||||
|
10.10+ |
|
Employment Agreement, dated October 1, 2013, between the Registrant and Raymond Huggenberger. |
|
S-1/A |
|
333-192605 |
|
10.10 |
|
12/23/13 |
|
|
|
|
|
|
|
|
|
|||||
|
10.11+ |
|
Employment Agreement, dated October 1, 2013, between the Registrant and Scott Wilkinson. |
|
S-1/A |
|
333-192605 |
|
10.11 |
|
12/23/13 |
|
|
|
|
|
|
|
|
|
|||||
|
10.12+ |
|
Employment Agreement, dated October 1, 2013, between the Registrant and Alison Bauerlein. |
|
S-1/A |
|
333-192605 |
|
10.12 |
|
12/23/13 |
|
|
|
|
|
|
|
|
|
|||||
|
10.13+ |
|
Employment Agreement, dated October 1, 2013, between the Registrant and Matt Scribner. |
|
S-1/A |
|
333-192605 |
|
10.13 |
|
12/23/13 |
|
|
|
|
|
|
|
|
|
|||||
|
10.14+ |
|
Employment Agreement, dated October 1, 2013, between the Registrant and Brenton Taylor. |
|
S-1/A |
|
333-192605 |
|
10.14 |
|
12/23/13 |
|
|
|
|
|
|
|
|
|
|||||
|
10.15 |
|
Amended and Restated Revolving Credit and Term Loan Agreement, dated October 12, 2012, between the Registrant and Comerica Bank, as amended. |
|
S-1/A |
|
333-192605 |
|
10.15 |
|
01/16/14 |
|
|
|
|
|
|
|
|
|
|||||
|
Exhibit |
|
|
|
Incorporated by Reference |
|||||||
|
|
Description of Exhibit |
|
Form |
|
File No. |
|
Exhibit |
|
Filing Date |
||
|
|
|
|
|
|
|
|
|||||
|
10.16 |
|
Security Agreement, dated October 12, 2012, between the Registrant and Comerica Bank. |
|
S-1/A |
|
333-192605 |
|
10.16 |
|
01/16/14 |
|
|
|
|
|
|
|
|
|
|||||
|
10.17 |
|
Multi-Purpose Commercial Building Lease, dated February 1, 2010, between the Registrant and Rockbridge Investments, L.P., as amended. |
|
S-1 |
|
333-192605 |
|
10.17 |
|
11/27/13 |
|
|
|
|
|
|
|
|
|
|||||
|
10.18 |
|
Lease Agreement, dated May 3, 2012, between the Registrant and Bayview (TX) Holding LLC. |
|
S-1 |
|
333-192605 |
|
10.18 |
|
11/27/13 |
|
|
|
|
|
|
|
|
|
|||||
|
10.19 |
|
License Agreement, dated July 23, 2007, between the Registrant and Air Products and Chemicals, Inc. |
|
S-1/A |
|
333-192605 |
|
10.19 |
|
12/23/13 |
|
|
|
|
|
|
|
|
|
|||||
|
10.20 |
|
Amendment to License Agreement, dated October 23, 2009, between the Registrant and Air Products and Chemicals, Inc. |
|
S-1 |
|
333-192605 |
|
10.20 |
|
11/27/13 |
|
|
|
|
|
|
|
|
|
|||||
|
10.21 |
|
Amendment No. 2 to License Agreement, dated October 4, 2010, between the Registrant and Air Products and Chemicals, Inc. |
|
S-1 |
|
333-192605 |
|
10.21 |
|
11/27/13 |
|
|
|
|
|
|
|
|
|
|||||
|
10.22 |
|
Amendment No. 3 to License Agreement, dated March 22, 2011, between the Registrant and Air Products and Chemicals, Inc. |
|
S-1 |
|
333-192605 |
|
10.22 |
|
11/27/13 |
|
|
|
|
|
|
|
|
|
|||||
|
10.23+ |
|
Management Carve-Out Bonus Award, dated July 1, 2012, between the Registrant and Alison Bauerlein. |
|
S-1/A |
|
333-192605 |
|
10.23 |
|
01/28/14 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.24+ |
|
Management Carve-Out Bonus Award, dated July 1, 2012, between the Registrant and Brenton Taylor. |
|
S-1/A |
|
333-192605 |
|
10.24 |
|
01/28/14 |
|
|
|
|
|
|
|
|
|
|||||
|
10.25+ |
|
Management Carve-Out Bonus Award, dated July 1, 2012, between the Registrant and Scott Wilkinson. |
|
S-1/A |
|
333-192605 |
|
10.25 |
|
01/28/14 |
|
|
|
|
|
|
|
|
|
|||||
|
10.26+ |
|
Management Carve-Out Bonus Award, dated July 1, 2012, between the Registrant and Byron Myers. |
|
S-1/A |
|
333-192605 |
|
10.26 |
|
01/28/14 |
|
|
|
|
|
|
|
|
|
|||||
|
10.27+ |
|
Management Carve-Out Bonus Award, dated July 1, 2012, between the Registrant and Matthew Scribner. |
|
S-1/A |
|
333-192605 |
|
10.27 |
|
01/28/14 |
|
|
|
|
|
|
|
|
|
|||||
|
23.1 |
|
Consent of BDO USA, LLP, Independent Registered Public Accounting Firm. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
23.2 |
|
Consent of Macias Gini & O’Connell LLP, Independent Registered Public Accounting Firm. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
23.3* |
|
Consent of Wilson Sonsini Goodrich & Rosati, Professional Corporation (included in Exhibit 5.1). |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
24.1 |
|
Power of Attorney (contained on signature pages to the Registration Statement on Form S-1)
|
|
|
|
|
|
|
|
|
|
*To be filed by amendment
+Indicates a management contract or compensatory plan or arrangement