

Company Overview January 2020 Exhibit 99.2 [INOGEN LOGO] OXYGEN. ANYTIME. ANYWHERE.

Notice regarding forward-looking statements These slides and the accompanying oral presentation (the “Presentation”) include forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, which are based on current expectations, estimates and projections based on information currently available to management. These forward-looking statements include, among others, statements relating to our preliminary, unaudited financial results as of January 13, 2020 and our guidance as of January 13, 2020, including our estimates of 2020 revenue; our expectations regarding decreasing reimbursement rates on both our rental revenue and the oxygen therapy market generally; the size and estimates of growth in the oxygen therapy market; our estimates concerning market penetration; our expectation regarding market headwinds and the impact on HME providers; our hiring expectations; product development; our expectations for positive cash flow and our needs for additional capital; and our expectations related to our recent acquisition of New Aera and the TAV technology. All statements other than statements of historical facts contained in this Presentation, including statements regarding our future results of operations and financial position, business strategy, prospective products, plans and objectives of management for future operations, and future results of current and anticipated products are forward-looking statements. Forward-looking statements are typically identified by words like “believe,” “anticipate,” “could,” “should,” “estimate,” “expect,” “intend,” “plan,” “project,” “will,” “forecast,” “budget,” “pro forma,” and similar terms. Forward-looking statements are subject to numerous risks and uncertainties that could cause actual results to differ materially from currently anticipated results, including but not limited to, risks arising from the possibility that we will not realize anticipated revenue; the impact of reduced reimbursement rates; the possible loss of key employees, customers, or suppliers; and intellectual property risks if we are unable to secure and maintain patent or other intellectual property protection for the intellectual property used in our products. In addition, our business is subject to numerous additional risks and uncertainties, including, among others, risks relating to market acceptance of our products; our ability to successfully launch new products and applications; competition; our sales, marketing and distribution capabilities; our planned sales, marketing, and research and development activities; interruptions or delays in the supply of components or materials for, or manufacturing of, our products; seasonal variations; unanticipated increases in costs or expenses; and risks associated with international operations. The known risks and uncertainties are described in detail under the caption “Risk Factors” and elsewhere in our Annual Report on Form 10-K for the year ended December 31, 2018. Additional information is also set forth in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2019 and our subsequent reports filed with the Securities and Exchange Commission, or SEC. Accordingly, our actual results may materially differ from our current expectations, estimates and projections. Unless otherwise specified herein, forward-looking statements represent our management’s beliefs and assumptions only as of our January 13, 2020 press release, and we undertake no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by applicable law. For more complete information about Inogen, Inc., please read our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, and other documents that we have filed and may file from time to time with the SEC. These documents can be obtained by visiting EDGAR on the SEC Web site at www.sec.gov. Certain financial data for 2019 included in this Presentation is based off our preliminary, unaudited estimates. Inogen is in the process of finalizing its results of operations for the year ended December 31, 2019, and therefore, final results are not yet available. These preliminary estimates are based solely upon information available to management as of the date of this Presentation. Inogen’s actual results may differ from these estimates due to the completion of its year-end closing procedures, final adjustments and developments that may arise between now and the time its financial results for the year ended December 31, 2019 are finalized. You should read Inogen’s audited consolidated financial statements for the year ended December 31, 2019 once they become available. [INOGEN LOGO] OXYGEN. ANYTIME. ANYWHERE 2.

Inogen POC offers freedom and mobility Stationary oxygen concentrator + Regular oxygen tank delivery Delivery Model Inogen Model 2.8 pounds (single battery) Run time up to 5 hours with a double battery A/C adaptable Conforms to all applicable FAA requirements Suitable for overnight use Portable oxygen concentrator (POC) Inogen® offers a single solution, freedom and independence Inogen One G4® [inogen logo] OXYGEN. ANYTIME. ANYWHERE.3
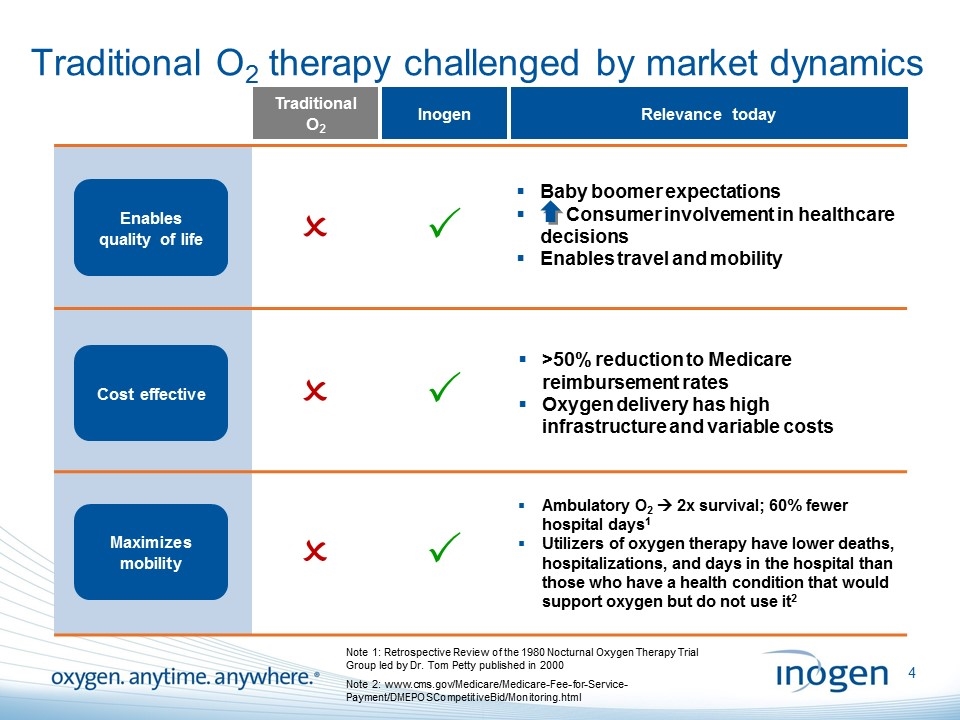
Traditional O2 therapy challenged by market dynamics Traditional O2 P Inogen Cost effective Maximizes mobility P P O O O Relevance today >50% reduction to Medicare reimbursement rates Oxygen delivery has high infrastructure and variable costs Ambulatory O2 à 2x survival; 60% fewer hospital days1 Utilizers of oxygen therapy have lower deaths, hospitalizations, and days in the hospital than those who have a health condition that would support oxygen but do not use it2 Baby boomer expectations Consumer involvement in healthcare decisions Enables travel and mobility Cost effective Enables quality of life Note 2: www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/DMEPOSCompetitiveBid/Monitoring.html Note 1: Retrospective Review of the 1980 Nocturnal Oxygen Therapy Trial Group led by Dr. Tom Petty published in 2000 [inogen logo] OXYGEN. ANYTIME. ANYWHERE.4
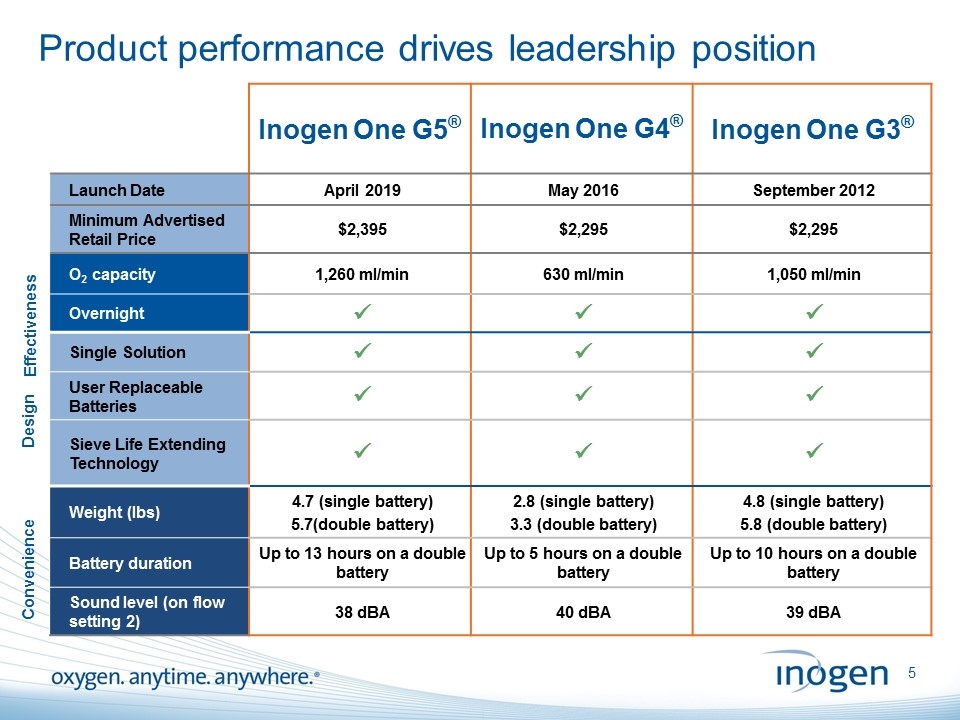
Product performance drives leadership position Inogen One G5® Inogen One G4® Inogen One G3® Launch Date April 2019 May 2016 September 2012 Minimum Advertised Retail Price $2,395 $2,295 $2,295 O2 capacity 1,260 ml/min 630 ml/min 1,050 ml/min Overnight ü ü ü Single Solution ü ü ü User Replaceable Batteries ü ü ü Sieve Life Extending Technology ü ü ü Weight (lbs) 4.7 (single battery) 5.7(double battery) 2.8 (single battery) 3.3 (double battery) 4.8 (single battery) 5.8 (double battery) Battery duration Up to 13 hours on a double battery Up to 5 hours on a double battery Up to 10 hours on a double battery Sound level (on flow setting 2) 38 dBA 40 dBA 39 dBA Effectiveness Design Convenience [inogen logo] OXYGEN. ANYTIME. ANYWHERE.5
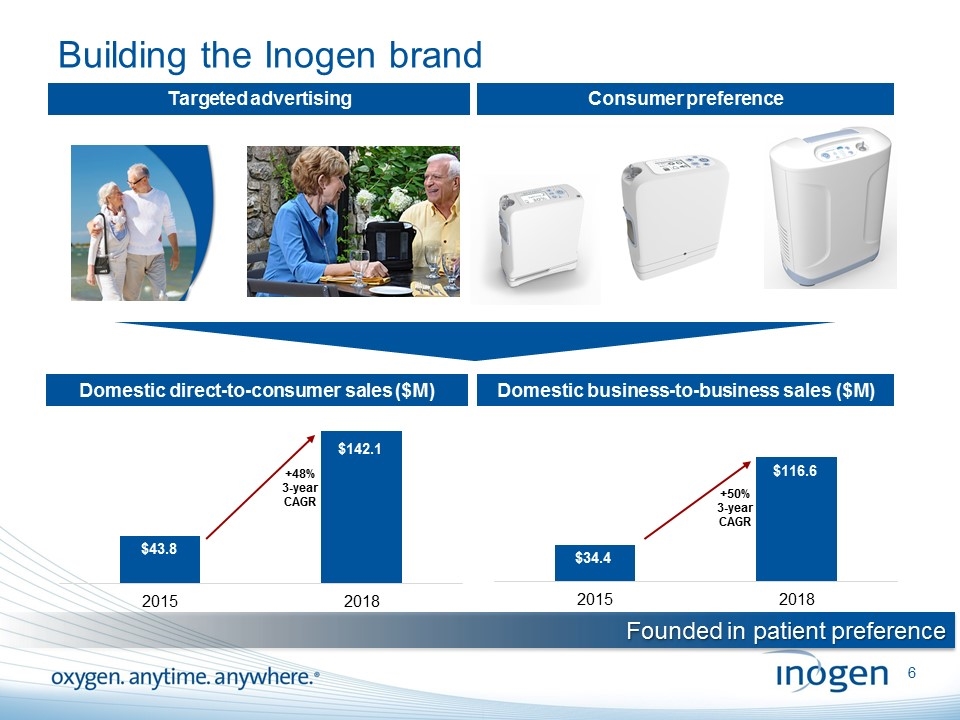
Building the Inogen brand Targeted advertising Consumer preference Domestic direct-to-consumer sales ($M) Domestic business-to-business sales ($M) +48% 3-year CAGR Founded in patient preference +50% 3-year CAGR [inogen logo] OXYGEN. ANYTIME. ANYWHERE.6
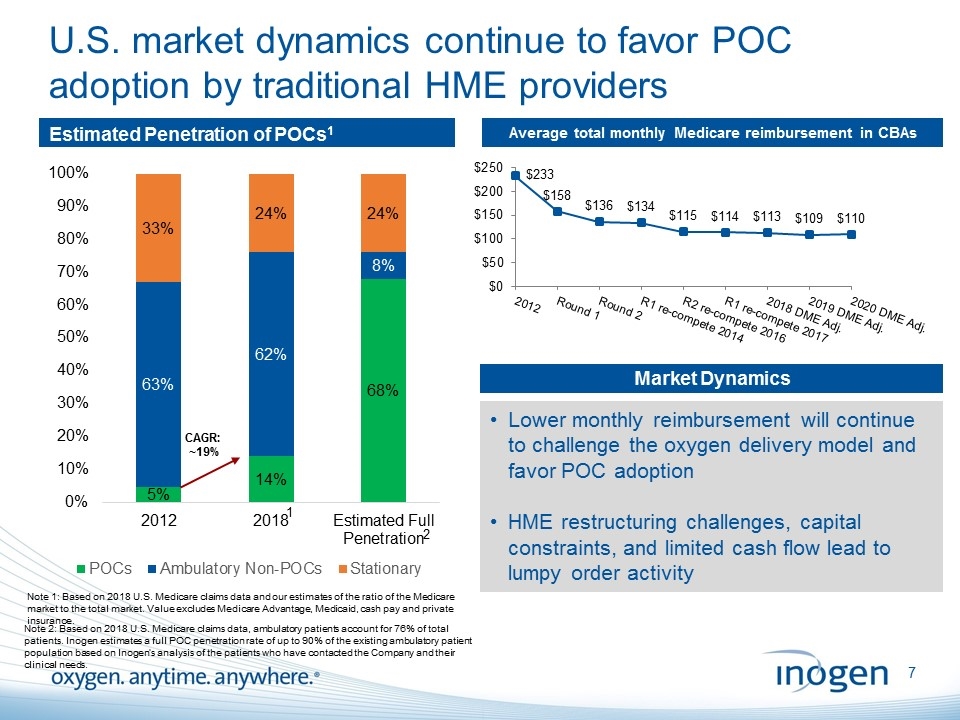
U.S. market dynamics continue to favor POC adoption by traditional HME providers Estimated Penetration of POCs1 Average total monthly Medicare reimbursement in CBAs Note 1: Based on 2018 U.S. Medicare claims data and our estimates of the ratio of the Medicare market to the total market. Value excludes Medicare Advantage, Medicaid, cash pay and private insurance. CAGR: ~19% 1 2 Note 2: Based on 2018 U.S. Medicare claims data, ambulatory patients account for 76% of total patients. Inogen estimates a full POC penetration rate of up to 90% of the existing ambulatory patient population based on Inogen’s analysis of the patients who have contacted the Company and their clinical needs. Market Dynamics Lower monthly reimbursement will continue to challenge the oxygen delivery model and favor POC adoption HME restructuring challenges, capital constraints, and limited cash flow lead to lumpy order activity [inogen logo] OXYGEN. ANYTIME. ANYWHERE.7
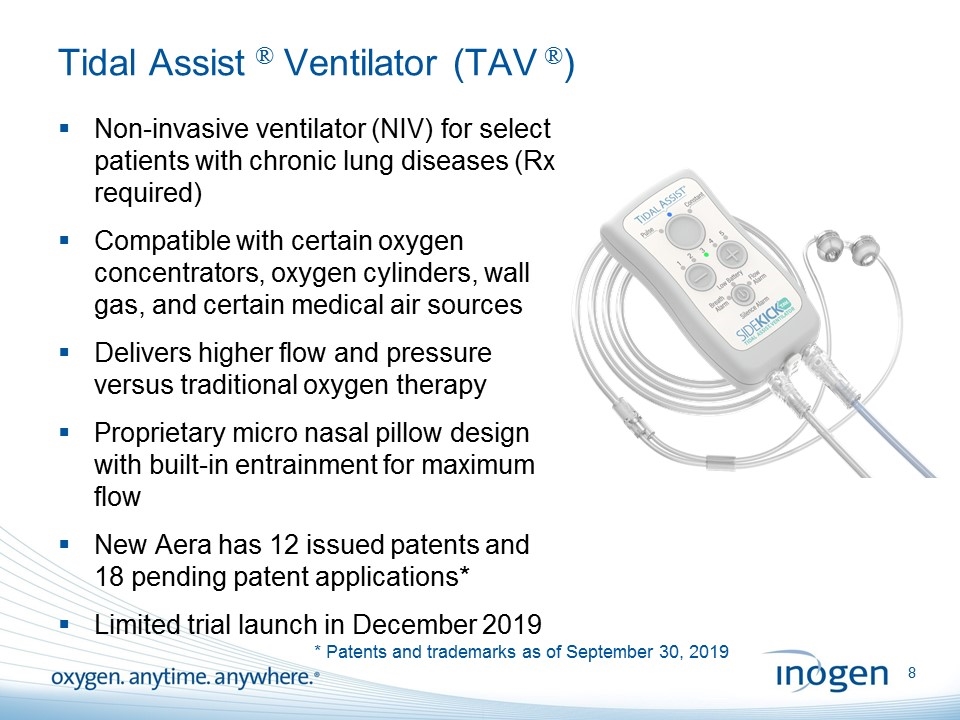
Tidal Assist ® Ventilator (TAV ®) Non-invasive ventilator (NIV) for select patients with chronic lung diseases (Rx required) Compatible with certain oxygen concentrators, oxygen cylinders, wall gas, and certain medical air sources Delivers higher flow and pressure versus traditional oxygen therapy Proprietary micro nasal pillow design with built-in entrainment for maximum flow New Aera has 12 issued patents and 18 pending patent applications* Limited trial launch in December 2019 * Patents and trademarks as of September 30, 2019 [inogen logo] OXYGEN. ANYTIME. ANYWHERE.8
Strategic value of New Aera Acquisition Complementary [inogen logo] OXYGEN. ANYTIME. ANYWHERE.9 Sales Opportunity Enhances Financial Profile Strengthens Technology Leadership Expansion into NIV Market
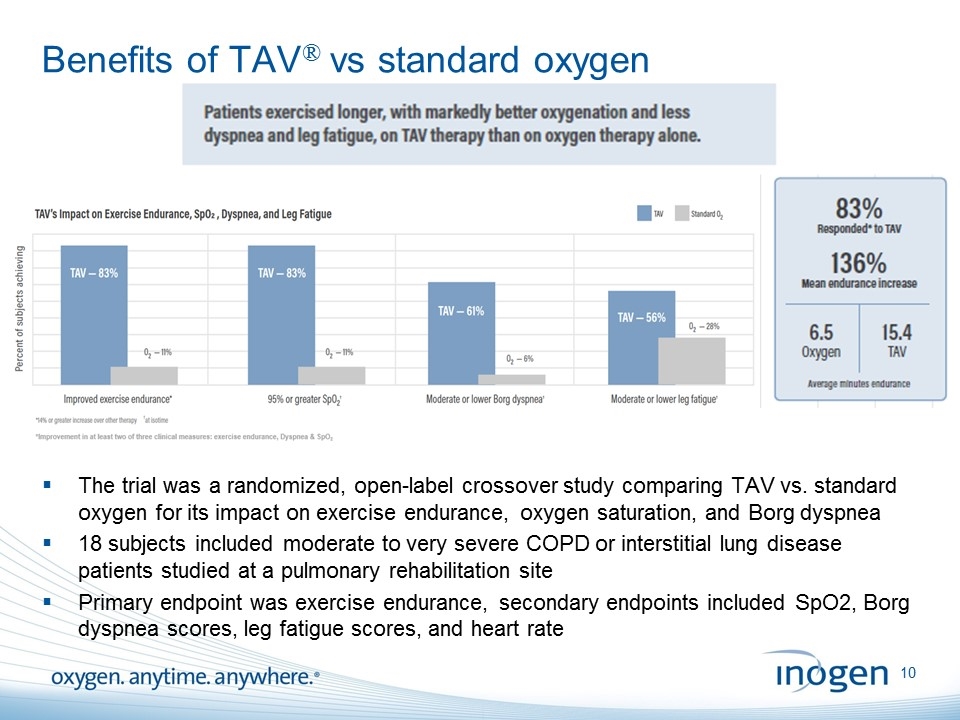
Benefits of TAV® vs standard oxygen The trial was a randomized, open-label crossover study comparing TAV vs. standard oxygen for its impact on exercise endurance, oxygen saturation, and Borg dyspnea 18 subjects included moderate to very severe COPD or interstitial lung disease patients studied at a pulmonary rehabilitation site Primary endpoint was exercise endurance, secondary endpoints included SpO2, Borg dyspnea scores, leg fatigue scores, and heart rate [inogen logo] OXYGEN. ANYTIME. ANYWHERE.10
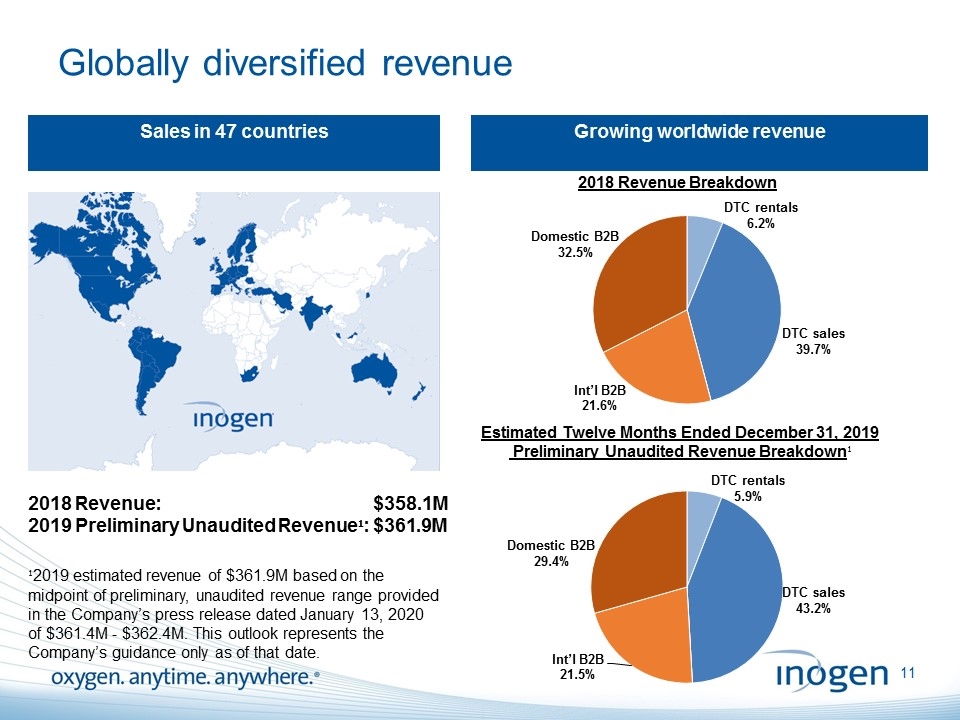
Globally diversified revenue Sales in 47 countries Growing worldwide revenue 2018 Revenue: $358.1M 2019 Preliminary Unaudited Revenue¹: $361.9M ¹2019 estimated revenue of $361.9M based on the midpoint of preliminary, unaudited revenue range provided in the Company’s press release dated January 13, 2020 of $361.4M - $362.4M. This outlook represents the Company’s guidance only as of that date. [inogen logo] OXYGEN. ANYTIME. ANYWHERE.11
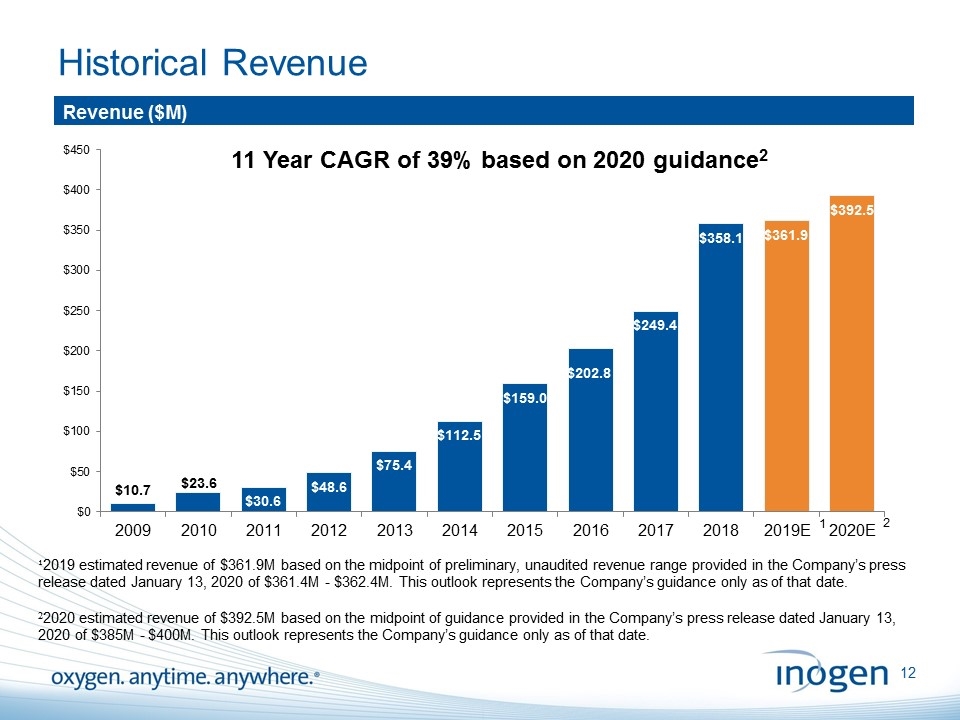
Historical Revenue Revenue ($M) 11 Year CAGR of 39% based on 2020 guidance2 ¹2019 estimated revenue of $361.9M based on the midpoint of preliminary, unaudited revenue range provided in the Company’s press release dated January 13, 2020 of $361.4M - $362.4M. This outlook represents the Company’s guidance only as of that date. 22020 estimated revenue of $392.5M based on the midpoint of guidance provided in the Company’s press release dated January 13, 2020 of $385M - $400M. This outlook represents the Company’s guidance only as of that date. 1 2 [inogen logo] OXYGEN. ANYTIME. ANYWHERE.12
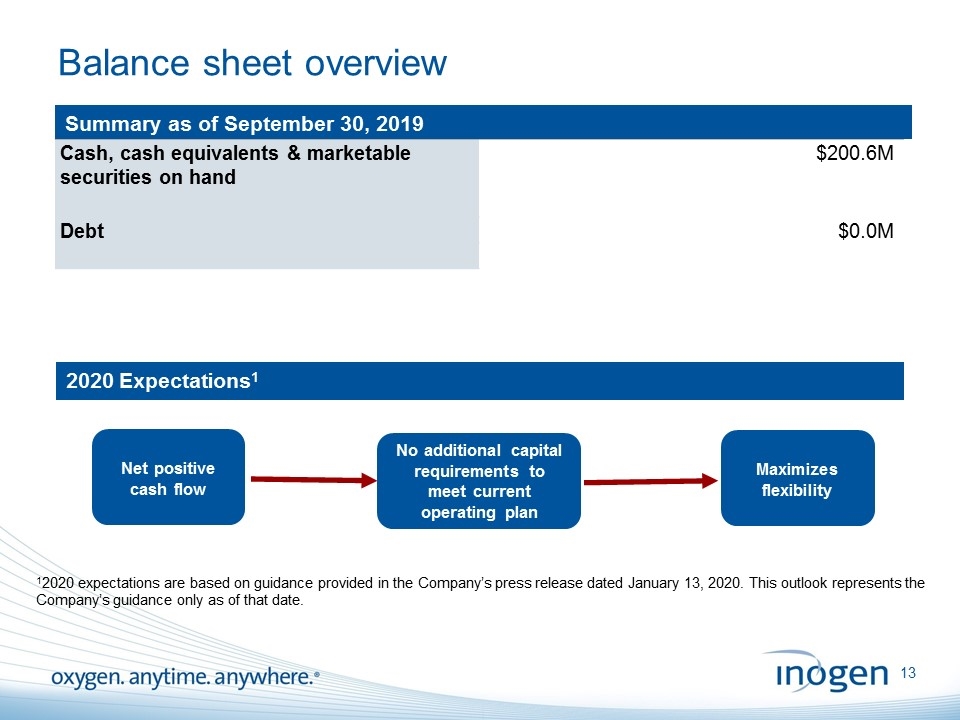
Balance sheet overview [Insert object title] Cash, cash equivalents & marketable securities on hand $200.6M Debt $0.0M No additional capital requirements to meet current operating plan Net positive cash flow Maximizes flexibility 2020 Expectations1 12020 expectations are based on guidance provided in the Company’s press release dated January 13, 2020. This outlook represents the Company’s guidance only as of that date. Summary as of September 30, 2019 [inogen logo] OXYGEN. ANYTIME. ANYWHERE.13
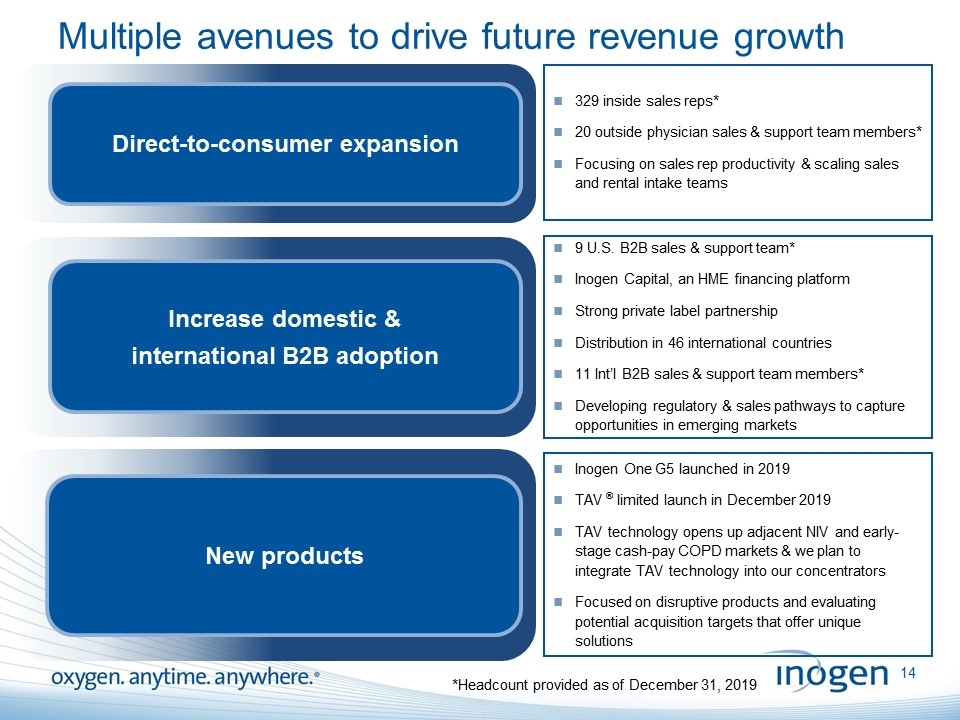
Multiple avenues to drive future revenue growth Direct-to-consumer expansion Increase domestic & international B2B adoption 329 inside sales reps* 20 outside physician sales & support team members* Focusing on sales rep productivity & scaling sales and rental intake teams 9 U.S. B2B sales & support team* Inogen Capital, an HME financing platform Strong private label partnership Distribution in 46 international countries 11 Int’l B2B sales & support team members* Developing regulatory & sales pathways to capture opportunities in emerging markets New products Inogen One G5 launched in 2019 TAV ® limited launch in December 2019 TAV technology opens up adjacent NIV and early-stage cash-pay COPD markets & we plan to integrate TAV technology into our concentrators Focused on disruptive products and evaluating potential acquisition targets that offer unique solutions *Headcount provided as of December 31, 2019 [inogen logo] OXYGEN. ANYTIME. ANYWHERE.14

Company highlights Market leader in large, global, underpenetrated market DTC model enables innovation and customer access Differentiated product portfolio with commitment to R&D Seasoned management team with proven track record Attractive financial profile [inogen logo] OXYGEN. ANYTIME. ANYWHERE.15

Proprietary and Confidential [inogen logo]

Supplemental Information January 2020 [inogen logo] OXYGEN. ANYTIME. ANYWHERE.
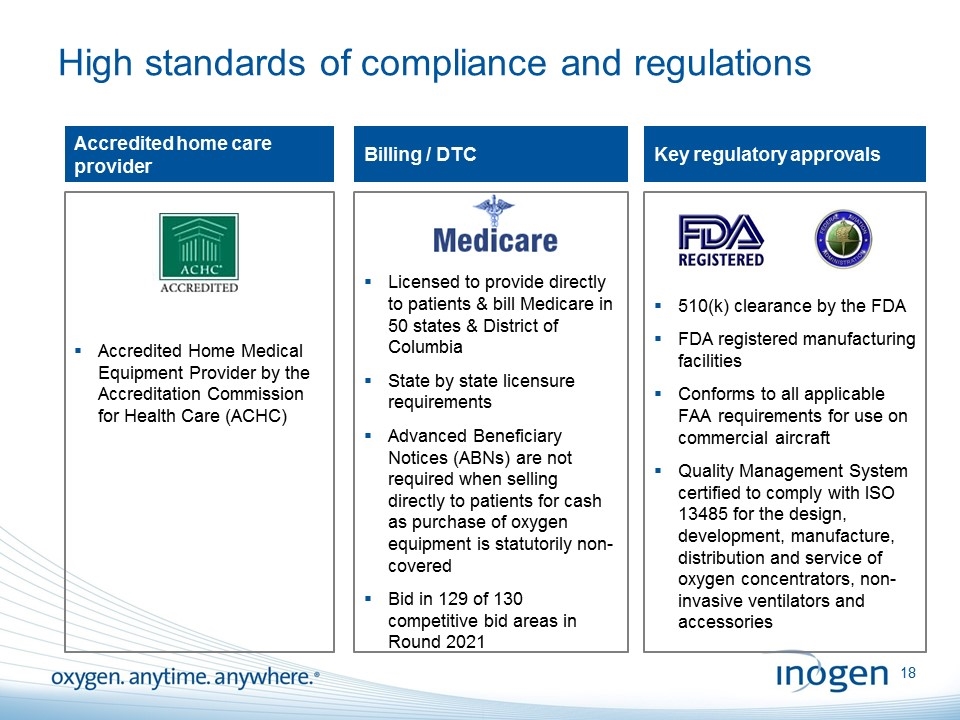
510(k) clearance by the FDA FDA registered manufacturing facilities Conforms to all applicable FAA requirements for use on commercial aircraft Quality Management System certified to comply with ISO 13485 for the design, development, manufacture, distribution and service of oxygen concentrators, non-invasive ventilators and accessories Licensed to provide directly to patients & bill Medicare in 50 states & District of Columbia State by state licensure requirements Advanced Beneficiary Notices (ABNs) are not required when selling directly to patients for cash as purchase of oxygen equipment is statutorily non-covered Bid in 129 of 130 competitive bid areas in Round 2021 Accredited Home Medical Equipment Provider by the Accreditation Commission for Health Care (ACHC) High standards of compliance and regulations Accredited home care provider Billing / DTC Key regulatory approvals [inogen logo] OXYGEN. ANYTIME. ANYWHERE.18
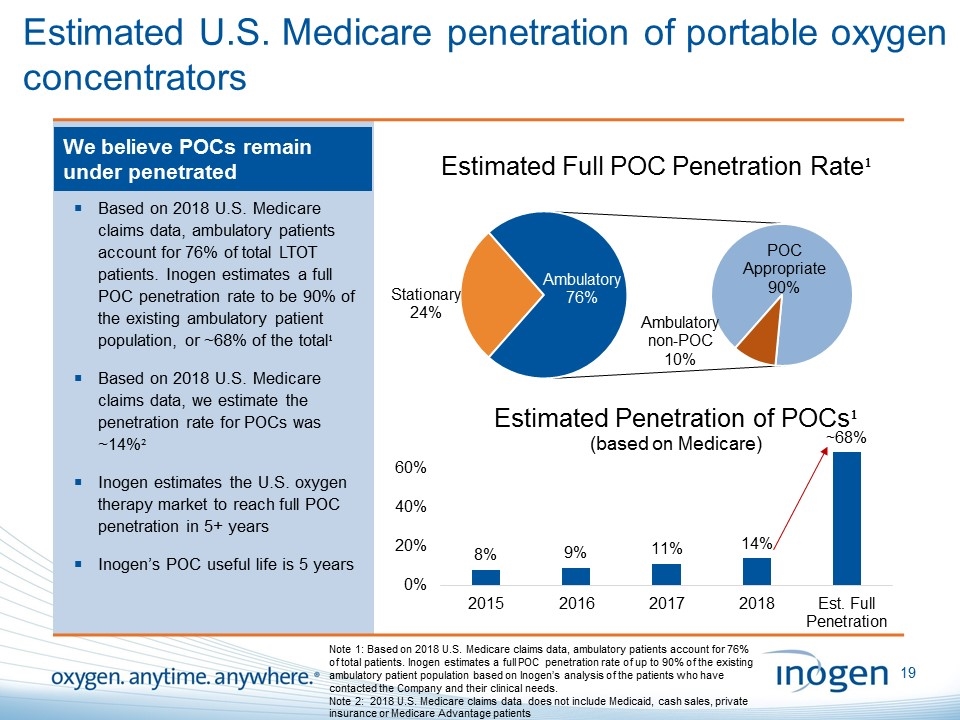
Estimated U.S. Medicare penetration of portable oxygen concentrators We believe POCs remain under penetrated Based on 2018 U.S. Medicare claims data, ambulatory patients account for 76% of total LTOT patients. Inogen estimates a full POC penetration rate to be 90% of the existing ambulatory patient population, or ~68% of the total¹ Based on 2018 U.S. Medicare claims data, we estimate the penetration rate for POCs was ~14%² Inogen estimates the U.S. oxygen therapy market to reach full POC penetration in 5+ years Inogen’s POC useful life is 5 years Note 1: Based on 2018 U.S. Medicare claims data, ambulatory patients account for 76% of total patients. Inogen estimates a full POC penetration rate of up to 90% of the existing ambulatory patient population based on Inogen’s analysis of the patients who have contacted the Company and their clinical needs. Note 2: 2018 U.S. Medicare claims data does not include Medicaid, cash sales, private insurance or Medicare Advantage patients [inogen logo] OXYGEN. ANYTIME. ANYWHERE.19
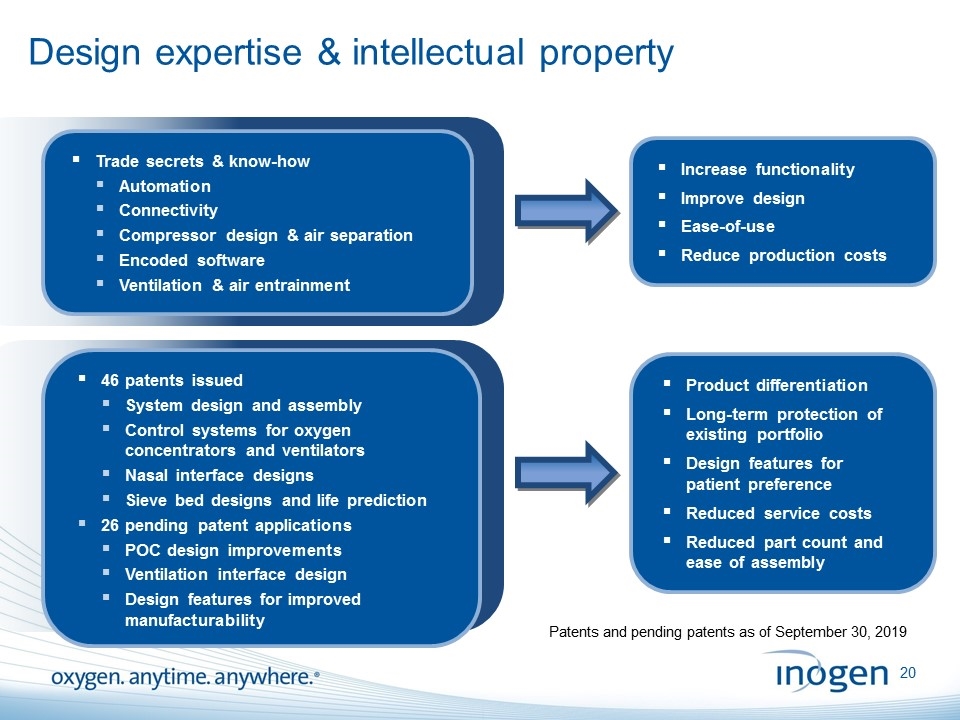
Design expertise & intellectual property 46 patents issued System design and assembly Control systems for oxygen concentrators and ventilators Nasal interface designs Sieve bed designs and life prediction 26 pending patent applications POC design improvements Ventilation interface design Design features for improved manufacturability Trade secrets & know-how Automation Connectivity Compressor design & air separation Encoded software Ventilation & air entrainment Product differentiation Long-term protection of existing portfolio Design features for patient preference Reduced service costs Reduced part count and ease of assembly Increase functionality Improve design Ease-of-use Reduce production costs Patents and pending patents as of September 30, 2019 [inogen logo] OXYGEN. ANYTIME. ANYWHERE.20
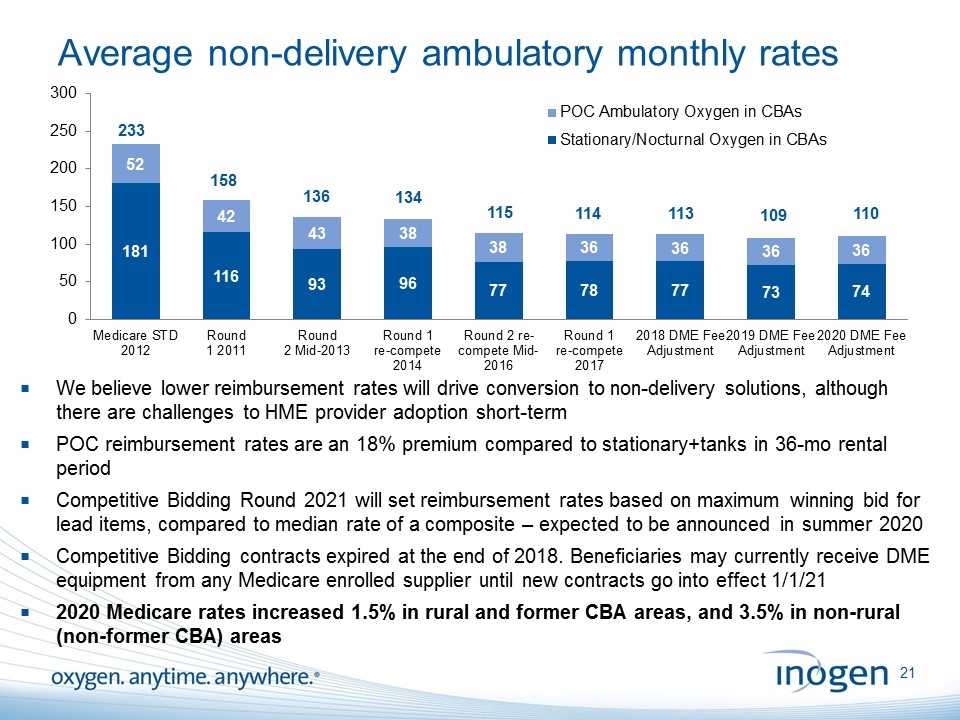
Average non-delivery ambulatory monthly rates 233 136 134 We believe lower reimbursement rates will drive conversion to non-delivery solutions, although there are challenges to HME provider adoption short-term POC reimbursement rates are an 18% premium compared to stationary+tanks in 36-mo rental period Competitive Bidding Round 2021 will set reimbursement rates based on maximum winning bid for lead items, compared to median rate of a composite – expected to be announced in summer 2020 Competitive Bidding contracts expired at the end of 2018. Beneficiaries may currently receive DME equipment from any Medicare enrolled supplier until new contracts go into effect 1/1/21 2020 Medicare rates increased 1.5% in rural and former CBA areas, and 3.5% in non-rural (non-former CBA) areas 158 115 114 113 109 110 [inogen logo] OXYGEN. ANYTIME. ANYWHERE.21
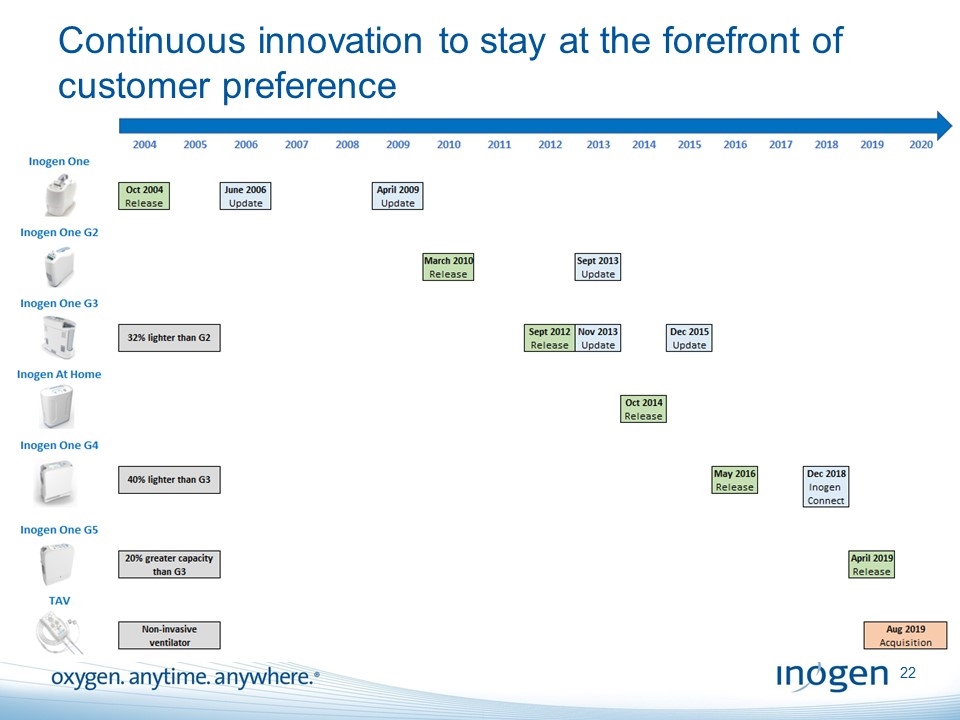
Continuous innovation to stay at the forefront of customer preference 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 inogen one inogen one g2 inogen one g3 inogen at home inogen one g4 inogen one g5 tav oct 2004 release June 2006 update April 2009 update march 2010 release sept 2013 update sept 2012 release nov 2013 update dec 2015 update oct 2014 release may 2016 release dec 2018 inogen connect April 2019 release aug 2019 acquisition 32% lighter then g2 40% lighter than g3 20% greater capacity than g3 non-invasive ventilator [inogen logo] OXYGEN. ANYTIME. ANYWHERE.22

A proven team built for success Ali Bauerlein Chief Financial Officer, Executive Vice President, Finance, Corporate Secretary & Corporate Treasurer Scott Wilkinson President, Chief Executive Officer, BOD Member Co-founder of Inogen with over 18 years experience in treasury, finance, accounting, risk management as well as strategic and tactical cost analysis and forecasting 30+ years of leadership with Johnson & Johnson, Kimberly-Clark, Invacare in operations, R&D, product management, sales & marketing Bart Sanford Executive Vice President, Operations 30+ years of manufacturing and operations leadership experience, with 18 years in Danaher Corporation (Cepheid, Molecular Devices, Fluke Corporation) Brenton Taylor Executive Vice President, Engineering Co-founder of Inogen with over 18 years of experience in medical device product development and manufacturing Successfully obtained 26 issued U.S. patents for POC development Byron Myers Executive Vice President, Sales & Marketing Co-founder of Inogen with over 18 years experience with direct responsibility for sales, marketing and product management operations MBA, UCSD Rady School of Management [inogen logo] OXYGEN. ANYTIME. ANYWHERE.23